Management of abnormal muscle tone: neurosurgical procedures to reduce spasticity
Evidence review A2
NICE Guideline, No. 119
Authors
National Guideline Alliance (UK).Management of abnormal muscle tone in adults aged 19 and over with cerebral palsy, including spasticity and associated movement disorders such as dystonia
Review question
A2 Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
Introduction
When aggravating factors are removed and enteral or intramuscular pharmacological agents have been tried to their maximum tolerated dosage, neurosurgical interventions, such as intrathecal baclofen therapy and selective dorsal rhizotomy, are available for spasticity management. Both procedures require anaesthetic, and have surgical and recovery risks. However, they also have the potential to reduce spasticity and pain and improve quality of life. The aim of this review question is to examine the effectiveness of these interventions, taking into account the burden of having surgery, follow up and potential adverse events, as well as patient and carer experience.
PICO table
Please see Table 1 for a summary of the Population, Intervention, Comparison and Outcome (PICO) characteristics of this review.
Table 1
Summary of the protocol (PICO table).
For full details see the review protocol in appendix A.
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual 2014. Methods specific to this review question are described in the review protocol in appendix A and for a full description of the methods see supplementary document C.
Declaration of interests were recorded according to NICE’s 2014 conflicts of interest policy from May 2016 until April 2018. From April 2018 onwards they were recorded according to NICE’s 2018 conflicts of interest policy. Those interests declared until April 2018 were reclassified according to NICE’s 2018 conflicts of interest policy (see Interests Register).
Clinical evidence
Included studies
Two randomised cross-over trials (number of participants, N=18; Albright 1991 and van Shaeybroeck 2000) and 6 before and after observational studies (N=99; Bertelli 2003, Gerszten 1997, Meythaler 2001, Motta 2011, Reynolds 2011 and Tassell-Ponche 2010) were included in the review. The clinical studies included in this evidence review are summarised in Table 2 and evidence from these is summarised in the clinical evidence profiles (Table 3 and Table 4).
Five studies were of long term continuous intrathecal baclofen infusion (Gerszten 1997, Meythaler 2001, Motta 2011, Tassell-Ponche 2010 and van Shaeybroeck 2000), 2 studies were randomised blinded comparisons of different doses of short term bolus injections of intrathecal baclofen (Albright 1991 and van Shaeybroeck 2000) and 2 studies concerned dorsal rhizotomy (Bertelli 2003 and Reynolds 2011).
The clinical studies included on this evidence review are summarised in Table 2 and evidence from these are summarised in the clinical evidence profile below (Table 3 and Table 4).
See also the literature search strategy in appendix B, study selection flow chart in appendix C, forest plots and dose comparison graph in appendix E and study evidence tables in appendix D.
Excluded studies
Studies excluded from this systematic review, with reasons for their exclusion, are provided in appendix K.
Table 2 provides a brief summary of the included studies.
Table 2
Summary of included studies.
See appendix D for the full evidence tables.
Quality assessment of clinical outcomes included in the evidence review
The clinical evidence profiles for this review question are presented in Table 3 and Table 4.
Table 3
Summary clinical evidence profile: Comparison 1: intrathecal baclofen pre-operative versus post-operative.
Table 4
Summary clinical evidence profile: Comparison 2: selective dorsal rhizotomy – pre-operative versus post-operative.
See appendix F for the full GRADE tables.
Economic evidence
Included studies
See supplementary material D for the economic evidence tables.
Excluded studies
See supplementary material D for the list of excluded studies.
Summary of studies included in the economic evidence review
Bensmail 2009 is a cost effectiveness study comparing intrathecal baclofen as a first-line strategy to current specific treatment options offered to patients with disabling spasticity. The study took a French public healthcare payer perspective and reported outcomes in terms of cost per success defined as increased patient and caregiver satisfaction and a decrease of at least one point on the Ashworth score. Effectiveness data was taken from historical databases which were not defined in the paper. The study population was for people with disabling spasticity and was not exclusive to people with cerebral palsy. Whilst the databases would include people with cerebral palsy the paper did not report the total number or proportion this group made up. Costs were taken from one retrospective resource utilisation study of 170 patients with disabling spasticity at 1 French hospital.
Sampson 2002 was a study looking at the change in QALYs and costs incurred with the use of intrathecal baclofen from pre-treatment on people with severe spasticity. The study took a UK NHS perspective and reported outcomes in terms of change in QALYs from baseline and total costs. Given this was a before and after type study no incremental costs were calculated between competing interventions. Effectiveness data for the study was taken from 1 meta-analysis of 17 comparative and non-comparative trials. The meta-analysis did not report the total number or proportion of patients with cerebral palsy (as some of the included studies did not report this) and it included trials in populations without cerebral palsy. This data was used by clinicians to estimate the baseline and change in the 5 dimensions of the EQ-5D and the impact on quality of life for the use of intrathecal baclofen. 3 categories of patients with different levels of disability were considered by the study: Category 1, bedbound patients experiencing severe spasm-related pain; Category 2, bedbound patients who were not in pain; Category 3, wheelchair users with moderate spasm related pain. Cost drivers were identified from discussion with clinicians and costs estimated using costing data from three UK centres.
Saulino 2015 compared the cost of care before and after intrathecal baclofen pump based on a retrospective analysis of commercial administrative claims data for people with severe spasticity [people with cerebral palsy (n=131), multiple sclerosis (n=124), and spinal cord injury (n=40)]. The costs considered were those to a private US healthcare payer and included all healthcare related costs. A 30 year time horizon was considered using decision analytical modelling to estimate costs over the remainder of the patient’s lifetime.
See appendix I for the full health economic evidence profiles.
Economic model
This question was not prioritised for economic modelling given that previous economic evidence was identified. Instead, a cost-description was undertaken to aid considerations of resource impact and cost effectiveness.
Resource impact
Selective dorsal rhizotomy (SDR)
Edwards 2010 was a detailed cost-analysis that gave a thorough understanding of the costs involved in SDR in adolescents. Those costs are reproduced in Table 5. For each patient, a data collection sheet was used to record all contacts with the hospital or one of its outreach services in schools and clinics in other Trusts. Contact episodes were separately identified as outpatient appointments, multidisciplinary team sessions, gait assessments, orthotics supplies, hospital admissions, surgical or other in-patient interventions, and admissions for physiotherapy top-up.
Table 5
Unit costs of selective dorsal rhizotomy treatment.
The cost of additional follow-up clinic visits was not included since all patients are followed up routinely post-surgery. The neurophysiological spinal monitoring equipment was also not included in the costing as it was treated as a sunk cost for other spinal surgeries.
They reported all the patient contacts for each group including musculoskeletal surgery. They found the number of outpatient visits showed no significant variation between groups. Non-SDR patients (n=4) underwent an average of 3 periods of surgery in total and SDR an average of 1.9, although the SDR patients (n=9) spent longer in hospital (83 days compared to 57.5 in the non-SDR group).
The cost data presented by Edwards 2010 was thorough and provides useful information. However, these are small patient numbers so it may be unreliable to compare the groups. Moreover, the costs were taken from procedures undertaken in adolescents which may not be reflective of adults. For example, the committee advised that a recovery on the ward would be reduced to 2 weeks for adults in clinical practice today. In order to provide a useful analysis for decision making, evidence on the long-term benefits and risks of treatment compared to the next best alternative are needed.
Intrathecal baclofen (ITB)
Sampson 2002 published a study on ITB in which detailed cost estimates were derived from 3 centres in the UK where the procedure was being performed. The costs included in the study were obtained in 1999 and have been converted to 2015/16 costs using the hospital and community health services pay and prices index uplift (Curtis PSSRU 2015) in Table 6.
Table 6
Cost of intrathecal baclofen reproduced from Sampson 2002.
The East Midlands Specialised Commissioning Group also produced detailed paediatric and adult costs for ITB treatment in 2009. They assumed the admission for the test dose usually takes 2 days whilst the admission for the implant usually takes an additional 5 days. The test dose, implant and refills were worked out using the contract code AB05Z (for intermediate pain procedures), at 2009/2010 prices. Those prices are presented alongside 2015/16 costs in Table 7.
Table 7
Cost of ITB treatment based on East Midlands commissioning policy 2009.
The total costs over 5 years are similar in the Sampson 2002 study and in the East Midlands Commissioning Policy; however, it is likely that the costs from the latter source are more accurate as costs were based on an HRG code, reflecting more recent UK practice. It is also important to note that the committee advised that the number of refills reported by those sources is overestimated as 2 to 3 refills a year are seen in UK clinical practice today.
Evidence statements
Clinical evidence statements
Comparison 1. intrathecal baclofen pre-operative versus post-operative
Critical outcomes
Walking
- Very low quality evidence from one before and after study including 24 people with cerebral palsy indicated no clinically significant improvement in the rates of household or community ambulation after four years of continuous baclofen infusion
Gross motor function
- Very low quality evidence from one before and after study including nine people with cerebral palsy indicated no clinically significant improvement in gross motor function after one year of continuous baclofen infusion.
Muscle tone
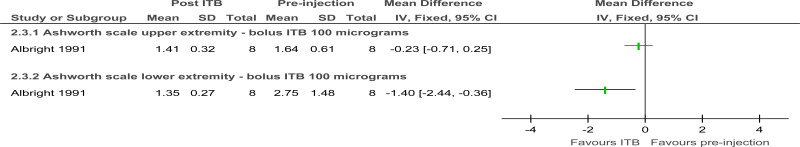
- Low quality evidence from 1 randomised study including 8 people with cerebral palsy and moderate or severe spasticity indicated bolus intrathecal injections of baclofen did not produce a clinically significant reduction in upper extremity muscle tone (at 4 hour follow-up.
- Low quality evidence from 1 randomised study including 8 people with cerebral palsy and moderate or severe spasticity indicated bolus intrathecal injections of baclofen led to a clinically significant reduction in lower extremity muscle tone (at 4 hour follow-up).
- Low quality evidence from 2 randomised studies including 18 people indicated higher dose bolus intrathecal injections of baclofen were more effective in lowering the muscle tone of people with cerebral palsy and moderate or severe spasticity
- Very low quality evidence from 2 before and after studies including 38 people with cerebral palsy who responded to trial bolus injections of intrathecal baclofen suggested continuous baclofen infusion via an implanted pump can maintain a clinically significant reduction in lower extremity muscle tone over one to five years of follow-up.
- Very low quality evidence from 1 before and after study including 13 people with cerebral palsy who responded to trial bolus injections of intrathecal baclofen suggested continuous baclofen infusion via an implanted pump can maintain a clinically significant reduction in upper extremity muscle tone over one year of follow-up.
Health related quality of life
- No evidence was found for this outcome
Important outcomes
Pain
- No evidence was found for this outcome
Adverse events
- Very low quality evidence about the rate of catheter or pump infections following implantation of baclofen infusion pumps was provided by 2 observational studies including 49 people followed for up to five years. Infections were reported by both studies at rates ranging from 4 to 8% over the period of follow-up.
- Very low quality evidence about the rate of catheter disconnection or breakage following implantation of baclofen infusion pumps was provided by 3 observational studies including 55 people followed for up to 5 years. Catheter disconnection or breakage was observed in all the studies, at rates ranging from 4 to 17% over the period of follow-up.
- Very low quality evidence about the rate of constipation following implantation of baclofen infusion pumps was provided by 2 observational studies including 38 people followed for up to 5 years. There was no comparison group in these studies so it was unclear whether constipation was more or less likely following pump implantation.
- Very low quality evidence about the rate of anxiety and depression following implantation of baclofen infusion pumps was provided by 1 observational study including 13 people followed for five years. There was no comparison group in this study so it was unclear whether anxiety and depression were more or less likely following pump implantation.
- Very low quality evidence about the rate of seizures following implantation of baclofen infusion pumps was provided by 1 observational study including 13 people followed for five years. There was no comparison group in this study so it was unclear whether seizures were more or less likely following pump implantation.
Satisfaction (patient or carer reported)
- No evidence was found for this outcome
Use of concurrent medications
- No evidence was found for this outcome
Comparison 2. Selective dorsal rhizotomy pre-operative versus post-operative
Critical outcomes
Walking
- Very low quality evidence from 1 before and after study including 21 people indicated no clinically significant improvement in self rated ambulatory ability five years after selective dorsal rhizotomy.
- Very low quality evidence from 1 before and after study including 7 people indicated a clinically significant improvement in the walking, running and jumping component of the GMFM scale five years after selective dorsal rhizotomy.
Gross motor function
- Very low quality evidence from 1 before and after study including 7 people indicated no improvement in the total GMFM score four months after selective dorsal rhizotomy.
- Very low quality evidence from 1 before and after study of 7 people with cerebral palsy and spastic hemiplegia indicated a clinically significant improvement in hand function 15 months after brachial plexus selective dorsal rhizotomy.
Muscle tone
- Low quality evidence from two before and after studies including 26 people with cerebral palsy and spasticity indicated that selective dorsal rhizotomy can produce a clinically significant reduction in muscle tone, at four to 15 months follow-up.
Health related quality of life
- Very low quality evidence from 1 observational study including 21 people with cerebral palsy indicated no clinically significant change in self-rated health related quality of life 5 years after selective dorsal rhizotomy.
Important outcomes
Pain
- Very low quality evidence from 1 observational study including 21 people with cerebral palsy indicated no clinically significant change in self-rated pain 5 years after selective dorsal rhizotomy.
Adverse events
- No evidence was found for this outcome
Satisfaction (patient or carer reported)
- No evidence was found for this outcome
Use of concurrent medications
- No evidence was found for this outcome
Health economic evidence statements
- One cost effectiveness analysis (Bensmail 2009) found that over 2 years, intrathecal baclofen dominated other established treatment patterns by providing greater effectiveness at a lower cost. The study showed a significantly lower average cost per success with ITB as a first-line strategy (€75,204/success versus €148,822/success; p<0.001). This analysis is partially applicable with potentially serious limitations, namely as the population was not exclusive to people with cerebral palsy and did not report outcomes in terms of QALYs. It is also unclear from the paper what clinical effectiveness data was used to inform the model as no values are reported in the paper. The study reported the mean results from 5000 PSA iterations. Deterministic results or deterministic sensitivity analyses of alternate assumptions were not reported.
- One cost utility analysis (Sampson 2002) estimated that the mean cost per QALY ranged from £6,900 to £12,790 over 5 years. Threshold analyses were reported which looked at the QALY gain needed to give mean costs of between £5000 and £25,000 per QALY. These were not comparative results with competing interventions and should be interpreted against cost per QALY thresholds with caution. This analysis is partially applicable as the population was not exclusive to people with cerebral palsy and NICE’s preferred discount rate was not applied. The evidence was associated with potentially serious limitations due to the limited sensitivity analysis reported.
- One cost analysis (Saulino 2015) found that at 30 years, intrathecal baclofen had a cumulative cost saving of $240,272 per patient equating to an annual saving of $8,009 compared with conventional treatment. This analysis is partially applicable as US costs will not be easily generalisable to the UK and no health related outcomes were estimated. The evidence was associated with potentially serious limitations as clinical effectiveness data was informed by a retrospective analysis of commercial administrative claims data rather than a systematic review of the literature. The study was funded by Medtronic Inc a manufacturer of intrathecal baclofen pumps.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The critical outcomes were gross motor function, walking, muscle tone and health related quality of life because neurosurgical procedures are primarily aimed at improving these outcomes. Important outcomes were reduction in pain and adverse events. Patient satisfaction and use of concurrent medications were also outcomes that the committee considered to be important. However, these outcomes were not reported.
The quality of the evidence
The quality of the evidence for this review was assessed using GRADE. The evidence for outcomes related to the effectiveness of intrathecal baclofen pumps and selective dorsal rhizotomy was very low to low quality. Overall this was due to the following general pattern common to the evidence related to intrathecal baclofen and selective dorsal rhizotomy:
- Although two blinded studies showed a dose response relationship between the dose of bolus intrathecal baclofen injections and muscle tone, the evidence for all outcomes from these studies was downgraded for indirectness as it came from the test dose using intrathecal injections rather than the dose following implantation of the pump. Intrathecal baclofen pumps would only be implanted after a response to a test dose and the results from these studies may therefore underestimate the effectiveness of this procedure.
- The level of evidence was also downgraded due to study design. This was because the majority of the evidence came from before and after observational studies: only two randomised dose comparison trials were included.
- The number of participants in each study was also very small due to the invasive nature of the treatments which led to wide confidence intervals and further downgrading of the evidence quality due to imprecision.
- One of the selective dorsal rhizotomy studies was agreed to be only partially applicable to the review question as it looked at brachial plexus dorsal rhizotomy to improve upper limb function rather than selective dorsal rhizotomy to improve lower limb function. Because only limited evidence was available the committee agreed to include this study but had little confidence in the findings.
The low quality of the evidence meant that strong recommendations for neurosurgical procedures could not be made and that the committee was not confident in the findings. They therefore based the recommendations on intrathecal baclofen and selective dorsal rhizotomy predominantly on their experience and expertise.
Although outcomes related to adverse events associated with intrathecal baclofen pumps, evidence were all rated as very low, they featured in the discussion of the committee and contributed to decision making. The committee agreed, based on their knowledge and experience, that neurosurgical treatments are associated with the reported adverse events (catheter or pump infections, constipation, anxiety or depression and seizures) because the surgical procedures are complex and invasive.
Due to the limited low quality evidence on selective dorsal rhizotomy the committee decided to both cross reference to the NICE interventional procedures guidance Selective dorsal rhizotomy for spasticity in cerebral palsy IPG373 (2010) as well as recommending further research on the use of selective dorsal rhizotomy.
Benefits and harms
The evidence showed that there are potentially serious adverse events associated with intrathecal baclofen pumps. The committee noted, based on their experience, that the most serious adverse events include pump-related complications (for example battery failure or catheter leakage), infections, and baclofen withdrawal or overdose. Even though serious adverse events were not reported in the studies for selective dorsal rhizotomy, the committee agreed that it is a complex neurosurgical procedure with likely serious risks. Based on their knowledge and experience, the committee agreed that there were more risks associated with surgery compared to enteral medication and therefore recommended that such procedures should only be considered when people on enteral or intramuscular pharmacological agents develop side effects, or when they are found to be ineffective, i.e. when other treatment options had been exhausted.
Based on their knowledge and experience, the committee noted that shared decision making between healthcare professionals and the person with spasticity and cerebral palsy (and their family or carer as appropriate) was an integral part of good service provision. Information about the benefits and risks associated with neurosurgical options should be provided to the adult with cerebral palsy as part of a multidisciplinary treatment strategy. The committee agreed that clear treatment goals need to be established prior to the procedure in order to assess its effectiveness according to individual needs and circumstances.
Due to the complex nature of treatment with intrathecal baclofen the committee noted that the adult with cerebral palsy will need sufficient information to make an informed choice and that this is not always consistently provided. A number of issues should be considered when providing information specifically related to this surgical procedure, such as the need for a test dose preimplantation, requirement of pump refill and regular follow-ups, the details of what the surgical procedure involves, and a review of their 24 hour postural needs.
The committee recognised that the response to intrathecal baclofen needs to be tested before the pump would be implanted. They therefore highlighted a couple of particular points about the test dose and how it would be administered. The committee did not want to be too detailed about dosage and how the testing would be carried out because this is described in the British National Formulary (BNF).
The committee considered, based on the evidence, that intrathecal baclofen therapy reduces muscle tone and this could therefore lead to improved motor function and health related quality of life. The committee agreed there were likely to be risks associated with intrathecal baclofen therapy (as described above). The committee recognised that some people with cerebral palsy make functional use of increased muscle tone that can be associated with spasticity, for example to help them to walk or to transfer from a sitting to standing position. For these people reduction in muscle tone could have a negative impact on certain motor functions and therefore this was highlighted in one of the recommendations
The response to the test dose should then be assessed and discussed with the adult with cerebral palsy to ensure that a pump is only implanted when a benefit is established in advance.
The uncertainty about the benefits and harms of selective dorsal rhizotomy meant that the committee could not recommend its use outside the context of a specialist multidisciplinary team (with the relevant expertise in the management of spasticity) approach to assessment. The committee noted that selective dorsal rhizotomy should not be considered in isolation but as part of the full range of treatment options. They were aware that there was related NICE guidance (NICE interventional procedure guidance on selective dorsal rhizotomy for spasticity in cerebral palsy) and cross-referenced to this. The committee noted that the NICE guideline on spasticity in under 19s recommends the collection of national outcome data for all patients assessed for selective dorsal rhizotomy and that a database for children was established and used for a study via NHS commissioning through evaluation which may help to inform future guidance.
The committee also agreed that there were specific issues and uncertainties that would need to be highlighted to the adult with cerebral palsy in relation to selective dorsal rhizotomy (for example irreversibility of the procedure or uncertainties about the long-term benefits) to allow them to make an informed choice.
Due to the limited evidence and the uncertainty around selective dorsal rhizotomy the committee decided to draft a research recommendation comparing it with continuous intrathecal baclofen pump treatment. The committee agreed that this is important because of the differences between the two procedures: selective dorsal rhizotomy is a one off surgical procedure that reduces sensory input to the sensory–motor reflex arcs in the spinal cord responsible for increased muscle tone by dividing some of the lumbar sensory nerve roots. Intensive physiotherapy is necessary for several months after the procedure particularly in patients who were previously able to walk and may have to learn different walking skills. It is a recommended NICE procedure usually offered to people with cerebral palsy and GMFCS level I-III, however the committee noted that most of the evidence comes from children under the age of 10. Intrathecal baclofen is a surgical procedure to implant an infusing pump allowing continuous delivery of baclofen into the cerebrospinal fluid of the spine. The pump requires ongoing refilling at least twice a year and further surgery to replace the pump at end of battery life (6.5 years). It was discussed that this procedure is a recognised NICE approved treatment usually offered to people with cerebral palsy and GMFCS level III-V. The committee recommended this research because they know that both selective dorsal rhizotomy and intrathecal baclofen are effective in reducing spasticity; however there is very little comparative safety or effectiveness data and a lack of studies of selective dorsal rhizotomy in the adult population.
Cost effectiveness and resource use
Three partially applicable economic evaluations were included in this review that assessed the cost effectiveness of intrathecal baclofen. Those analyses were associated with minor to potentially serious limitations, but all three evaluations concluded intrathecal baclofen was a cost effective treatment for spasticity as intrathecal baclofen provided additional benefits to outweigh its high cost. The committee acknowledged the high cost to administer and maintain intrathecal baclofen and stated that a stepwise approach to management would be taken by using the least expensive and least invasive options first. Combined with the clinical evidence that found intrathecal baclofen to reduce muscle tone, the committee concluded there was good quality evidence that recommending intrathecal baclofen would be cost effective.
The committee also agreed that referral to a tone or spasticity management service offering continuous pump-administered intrathecal baclofen therapy should be considered only for adults who still have difficulties with spasticity despite other treatment. Targeted referral and assessment by specialists would minimise the downstream costs to manage decreases in function.
Before intrathecal baclofen pumps are implanted, the committee reiterated that a test dose or doses (intrathecal baclofen given to the person by lumbar puncture or through a spinal catheter) should be provided to assess the potential effects on symptoms and function. The committee felt the cost of the test dose was justifiable as it can pre-empt treatment failure and reduce the number of people who would need additional procedures to remove the implant.
No economic evaluations were identified that assessed selective dorsal rhizotomy, but the high cost of the procedure was reported by Edwards 2010. The committee weighed up the clinical evidence from 2 before and after studies that found selective dorsal rhizotomy to reduce muscle tone, but concluded that there was not enough high quality evidence to recommend selective dorsal rhizotomy as a cost effective use of resources. Instead a research recommendation was prioritised to compare selective dorsal rhizotomy and intrathecal baclofen in adults with cerebral palsy.
Other factors the committee took into account
The committee recognised that there was an interventional procedure guideline on Selective dorsal rhizotomy for spasticity in cerebral palsy IPG373 (2010) and cross-referenced to this guideline in the recommendation.
References
Albright 1991
Albright, A.L., Cervi, A., Singletary, J., Intrathecal baclofen for spasticity in cerebral palsy, JAMA, 265, 1418–1422, 1991 [PubMed: 1999883]Bensmail 2009
Bensmail, D, Ward, Ab, Wissel, J, Motta, F, Saltuari, L, Lissens, J, Cros, S, Beresniak, A, Cost-effectiveness modeling of intrathecal baclofen therapy versus other interventions for disabling spasticity (Structured abstract), Neurorehabilitation and Neural Repair, 23, 546–552, 2009 [PubMed: 19228818]Bertelli 2003
Bertelli, J. A., Ghizoni, M. F., Rodrigues Frasson, T., Fernandes Borges, K. S., Brachial plexus dorsal rhizotomy in hemiplegic cerebral palsy, Hand Clinics, 19, 687–699, 2003 [PubMed: 14596559]Gerszten 1997
Gerszten, P.C., Albright, A.L., Barry, M.J., Effect on ambulation of continuous intrathecal baclofen infusion, Pediatric Neurosurgery, 27, 40–44, 1997 [PubMed: 9486835]Meythaler 2001
Meythaler, J.M., Guin-Renfroe, S., Law, C., Grabb, P., Hadley, M.N., Continuously infused intrathecal baclofen over 12 months for spastic hypertonia in adolescents and adults with cerebral palsy, Archives of Physical Medicine & Rehabilitation, 82, 155–161, 2001 [PubMed: 11239304]Motta 2011
Motta, F., Antonello, C.E., Stignani, C., Intrathecal baclofen and motor function in cerebral palsy, Developmental Medicine and Child Neurology, 53, 443–448, 2011 [PubMed: 21480874]Reynolds 2011
Reynolds, M.R., Ray, W.Z., Strom, R.G., Blackburn, S.L., Lee, A., Park, T.S., Clinical outcomes after selective dorsal rhizotomy in an adult population, World Neurosurgery, 75, 138–144, 2011 [PubMed: 21492678]Sampson 2002
Sampson, F. C., Hayward, A., Evans, G., Morton, R., Collett, B., Functional benefits and cost/benefit analysis of continuous intrathecal baclofen infusion for the management of severe spasticity, Journal of Neurosurgery, 96, 1052–1057, 2002 [PubMed: 12066906]Saulino 2015
Saulino, M., Guillemette, S., Leier, J., Hinnenthal, J., Medical cost impact of intrathecal baclofen therapy for severe spasticity, Neuromodulation, 18, 141–149, 2015 [PubMed: 25145312]Tasseel Ponche 2010
Tasseel Ponche, S., Ferrapie, A. L., Chenet, A., Menei, P., Gambart, G., Menegalli Bogeli, D., Perrouin Verbe, B., Gay, S., Richard, I., Intrathecal baclofen in cerebral palsy. A retrospective study of 25 wheelchair-assisted adults, Annals of Physical & Rehabilitation Medicine, 53, 483–98, 2010 [PubMed: 20829144]Van Schaeybroeck 2000
Van Schaeybroeck, P., Nuttin, B., Lagae, L., Schrijvers, E., Borghgraef, C., Feys, P., Intrathecal baclofen for intractable cerebral spasticity: a prospective placebo-controlled, double-blind study, Neurosurgery, 46, 603–9; discussion 609–12, 2000 [PubMed: 10719857]
Appendices
Appendix A. Review protocols
Review protocol for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
Table 8. Review protocol for neurosurgical procedures for spasticity (PDF, 372K)
Appendix B. Literature search strategies
Literature search strategies for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
This appendix is a combined search strategy and will be the same for all the evidence reviews for the A review questions as listed below:
- A1: Which pharmacological treatments for spasticity (for example, enteral baclofen, tizanidine, diazepam, cannabinoids, and botulinum toxin injections) are most effective for improving motor function, participation and quality of life in adults with cerebral palsy?
- A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
- A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB)) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
Database: Medline & Embase (Multifile)
Database(s): Embase 1974 to 2018 March 22, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to Present
Database: Cochrane Library
Database: Web of Science
Appendix C. Clinical evidence study selection
Clinical evidence study selection for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
Figure 1. Flow diagram of clinical article selection for neurosurgery for spasticity review
Appendix D. Clinical evidence tables
Clinical evidence tables for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
Table 12. Studies included in the evidence review for neurosurgical procedures for spasticity (PDF, 369K)
Appendix E. Forest plots
Forest plots for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
Comparison 1. Intrathecal baclofen, post versus pre-operative outcomes
Figure 2. Walking after 4 years of continuous infusion ITB versus pre-operative
Figure 3. Gross motor function after 1 year of continuous infusion ITB versus pre-operative
Figure 5. Tone 4 hours after 100 micrograms ITB bolus versus pre-injection
Figure 6. Tone after 1 to 4 years of continuous infusion ITB versus pre-operative
Comparison 2. Selective dorsal rhizotomy, post versus pre-operative outcomes
Figure 8. Walking up to 5 years after selective dorsal rhizotomy versus pre-operative
Figure 9. Gross motor function 4 months after selective dorsal rhizotomy versus pre-operative
Figure 10. Hand function 15 months after selective dorsal rhizotomy versus pre-operative
Figure 11. Tone 4 to 15 months after selective dorsal rhizotomy versus pre-operative
Figure 12. Quality of life up to 5 years after selective dorsal rhizotomy versus pre-operative
Figure 13. Pain up to 5 years after selective dorsal rhizotomy versus pre-operative
Appendix F. GRADE tables
GRADE tables for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
Appendix G. Economic evidence study selection
Economic evidence study selection for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
See supplementary material D for the economic evidence study selection.
Appendix H. Economic evidence tables
Economic evidence tables for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
See supplementary material D for the economic evidence tables.
Appendix I. Health economic evidence profiles
Health economic evidence profiles for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
See supplementary material D for the economic evidence profiles.
Appendix J. Health economic analysis
Health economic analysis for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
No economic analysis was included in this review.
Appendix K. Excluded studies
Clinical and economic lists of excluded studies for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
Clinical studies
Table 15. Excluded clinical studies for neurosurgical procedures for spasticiy (PDF, 473K)
Economic studies
See supplementary material D for the excluded clinical studies.
Appendix L. Research recommendations
Research recommendations for review question A2: What is the effectiveness and cost effectiveness of selective dorsal rhizotomy compared to continuous intrathecal baclofen pump to reduce spasticity in adults with cerebral palsy?
Appendix M. Health Economic Quality Assessment
Health economic quality assessment for review question A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
Final
Evidence reviews
These evidence reviews were developed by the National Guideline Alliance, hosted by the Royal College of Obstetricians and Gynaecologists
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.