8.1.19.1. Economic evidence – literature review
An economic evaluation should ideally compare all relevant alternatives. No applicable studies of good enough methodological quality were identified comparing all interventions of interest –vitamin D or vitamin D analogues, potent or very potent corticosteroids, coal tar, dithranol and retinoids – in the treatment of patients with mild to moderate chronic plaque psoriasis.
Three studies18,37,289 were identified that included two or more of the relevant comparators. These are summarised in the economic evidence profile below ( and ). See also the full study evidence tables in Appendix I.
Calcipotriol versus short contact dithranol – Economic study characteristics.
Calcipotriol versus short contact dithranol – Economic summary of findings.
Six studies were selectively excluded, four due to very serious methodological limitations110,140,234,359 and two due to the availability of more applicable economic evidence21,310. Reasons for their exclusion are provided in Appendix G.
Ashcroft and colleagues present a simple decision tree analytic model to explore the relative cost effectiveness of topical calcipotriol and short contact dithranol. Caution should be exercised when interpreting the results of this study as it is unclear if the best possible sources were used to inform the parameters, and the short time horizon means that the costs of treatment failure may have not been fully accounted for.
Ashcroft et al. did not perform a quality of life assessment which limits its usefulness in determining cost effectiveness of the interventions studied. The shows the results of Ashcroft et al., with estimates of the possible incremental cost effectiveness ratio over a 1-year time horizon had quality of life measurements been incorporated. The ICERs presented below show that if utility gains of 0.03 or 0.09 are assumed (based on estimates used by other authors37,289 in the economic review) the additional cost of calcipotriol is very unlikely to be offset by the additional benefits associated with this treatment.
Economic summary of Ashcroft et al. findings with quality of life incorporated.
identified had potentially serious limitations with their chosen methodology. Bottomley and colleagues used an NHS provider perspective and was directly applicable, but is limited by the method used to generate estimates of treatment effect. The authors used performed an unadjusted indirect comparison which may introduce bias. The sensitivity analyses conducted by Bottomley et al. provide some indication that once daily product containing calcipotriol monohydrate and betamethasone dipropionate may be a cost effective strategy provided that the difference in utility between baseline and that experienced on the waiting list is small (i.e. 0.075). Interestingly, Bottomley and colleagues found concurrent but separate treatment with vitamin D or vitamin D analogue and potent corticosteroids to be the most expensive strategy and provided the least QALYs.
Vitamin D or vitamin D analogues vs potent corticosteroids vs combined and concurrent vitamin D or vitamin D analogues and potent corticosteroids (one applied in the morning and one in the evening) - Economic summary of findings.
Oh and colleagues compared calcipotriol and different durations of clobetasol after first line treatment of a potent corticosteroid (betamethasone valerate) failed. Their evidence suggests that where the needed dosage and length of treatment of calcipotriol is similar or less than the ultra high potency corticosteroid clobetasol propionate, then calcipotriol might be the more cost effective second line treatment, however its incremental cost effectiveness compared to 2 weeks of very potent steroid was over the NICE £20,000 per QALY threshold. In a second analysis, they found that calcipotriol performed better as a second line treatment for psoriasis which had returned following prior treatment with betamethasone valerate or other agents, with increased utility due to lower side effects compared to fluocinonide.
8.1.19.2. Economic evidence – original economic analysis
The review of clinical evidence for topical therapies used in the treatment of individuals with mild to moderate plaque psoriasis showed that there were a wide variety of options – emollients, tars, dithranol, retinoids, corticosteroids (potent and very potent), vitamin D or vitamin D analogues and combination products – each associated with certain advantages and disadvantages. The results of the network meta-analysis suggested that some interventions, such as combined or concurrent vitamin D analogue and potent corticosteroid, were more likely to induce clearance or near clearance than others. Given that these combined and concurrent application strategies carry additional cost compared to both their individual constituent parts and compared to other topical alternatives, it was important to consider whether these additional costs are justified by additional health benefits in terms of improved quality of life.
The choice of which topical therapy to offer patients with mild to moderate psoriasis in primary care was identified as among the highest economic priorities by the GDG because the greatest proportion of psoriasis patients are managed at this point in the care pathway. Even if the unit costs of the interventions are quite modest, the population affected is relatively large; therefore the health economic impact of any recommendation is likely to be substantial.
Three cost-effectiveness analyses were identified in the published literature, but each had methodological limitations that called its conclusions into question. The analysis by Ashcroft and colleagues18 was based on only one trial and included only two of the interventions of interest (dithranol and calcipotriol). The analysis by Oh and colleagues289 was quite old and had analysed economic outcomes for different lines of treatment within separate models each having different comparators, thus making it difficult to identify a clearly cost-effective sequence of topical therapies. The analysis by Bottomley and colleagues,37 although the most applicable of the included studies, used an unadjusted indirect comparison to inform the treatment effect estimates, which likely overestimated the effectiveness of some interventions and underestimated the effectiveness of others. Bottomley and colleagues also did not include all the possible comparators of interest. Due to the methodological limitations of the published economic analyses, there was still substantial uncertainty as to which topical therapy or therapies represented the best value for NHS resources. In order to reduce this uncertainty, an original cost-effectiveness analysis was undertaken by the guideline health economist in collaboration with the GDG. Below is a summary of the analysis that was undertaken. For full details please see Appendix M: Cost-effectiveness analysis.
8.1.19.3. Methods
An analysis was undertaken to evaluate the relative cost-effectiveness of different topical therapy sequences used in the treatment of individuals with mild to moderate chronic plaque psoriasis. A Markov model was used to estimate 12-month costs and quality-adjusted life years (QALYs) from a current UK NHS and personal social services perspective. A 12-month time horizon was considered clinically relevant and sufficiently long enough to capture important costs and consequences of first-line treatment in primary care. Uncertainty was explored through probabilistic analysis and sensitivity analysis. The performance of alternative treatment sequences was estimated using incremental cost-effectiveness ratios (ICERs), defined as the added cost of a given strategy divided by its added benefit compared with the next most expensive strategy. A threshold of £20,000 per QALY gained was used to assess cost-effectiveness.
The aim of the analysis was to identify the most cost-effective sequence of first, second and third line topical therapies. It was important to model sequences given that most patients will commence treatment with one topical and then try others before moving on to more intensive treatments such as phototherapy and/or systemic therapy. In all, 118 sequences were compared in the base case analysis. presents the list of possible first, second and third line treatments which may be combined in a sequence.
All possible sequences of first, second and third line interventions.
The following conditions were placed on the sequences, ensuring that they represented logical clinical practice:
Concurrent treatment with vitamin D or vitamin D analogue and potent corticosteroid (one applied in the morning and one in the evening) would not come after a failure of once daily combined product containing calcipotriol monohydrate and betamethasone dipropionate;
Once daily treatment with a given topical would not come after a failure of twice daily treatment with the same topical;
Once daily treatment with potent steroid or vitamin D or vitamin D analogue would not come after concurrent treatment with vitamin D or vitamin D analogue and potent corticosteroid (one applied in the morning and one in the evening) or once daily combined product containing calcipotriol monohydrate and betamethasone dipropionate;
No strategy could include potent corticosteroids among all three lines of treatment (including as part of concurrent vitamin D or vitamin D analogues and potent corticosteroid (one applied in the morning and one in the evening) or combined product containing calcipotriol monohydrate and betamethasone dipropionate).
Most comparators focus on evaluating a trial of three different treatments before referral for specialist review, but the GDG was also interested in whether earlier escalation of care might be more cost-effective. To test this, strategies have also been combined into two-treatment sequences with referral following a failure of second line treatment.
Due to the unacceptability of dithranol and coal tar as routine treatments (difficult application, risk of staining, strong and unpleasant odours, etc), these treatments were reserved for third line treatment only. This reflects their current placement in primary care given the availability of more acceptable and effective topicals such as those being compared as first and second line topicals. In a series of sensitivity analyses, other restrictions were placed on the potential sequences, namely due to concerns about the safety of continued use of potent corticosteroids.
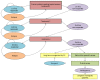
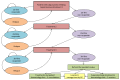
The structure of the model developed by the NCGC was adapted from the model developed by Bottomley and colleagues37 and was validated by the GDG as a reasonable reflection of current clinical practice. The Markov model and how patients move through the pathway is illustrated in . Key model assumptions (these are discussed in more detail in the full write-up in Appendix M):
Markov model of treatment with topical therapy.
All hypothetical patients commence treatment with a given topical and experience one of two outcomes after 4 or 8 weeks:
Patients who respond stop treatment and they either maintain response in the absence of treatment or they relapse.
Patients who do not respond to a given topical after 8 weeks of treatment are assumed to return to their GP and receive a prescription for an alternative topical therapy.
Patients can receive up to three different topical therapies before being referred by the GP to a specialist review in an outpatient dermatology clinic where second-line treatment options could be considered.
Some proportion of these referred patients will be kept on topical therapies, receive support and advice at the review consultation and be discharged back to their GP for long-term management.
The remaining proportion undergo a course of phototherapy:
- –
If they respond to phototherapy they are then discharged to their GP for long-term management.
- –
If they do not respond to phototherapy they continue to be managed by a specialist.
Movement between various health states is governed by transition probabilities, derived from the systematic review of clinical effectiveness data. Thirteen 4-week cycles were modelled, resulting in a 1-year time horizon for the analysis, with a half-cycle correction applied.
Model inputs were based on the clinical effectiveness review undertaken for the guideline, other published data and expert opinion where required. These are described in full in the technical report in Appendix M. All model inputs and assumptions were validated by the GDG.
8.1.19.4. Results
This analysis found that, given a NICE willingness-to-pay threshold of £20,000 per QALY gained, the most cost-effective strategy is likely to be one of starting with twice daily potent corticosteroid and moving to concurrent potent corticosteroid and vitamin D or vitamin D analogue (one applied in the morning and one in the evening) and then twice daily coal tar. This strategy was also the least costly strategy among the 118 modelled. Base case results for non-dominated and non-extendedly dominated strategies are presented .
Incremental analysis of base case results – psoriasis of trunk and limbs.
Results showed that starting with concurrent potent corticosteroid and vitamin D or vitamin D analogue (one applied in the morning and one in the evening) and switching to twice daily potent corticosteroid and then twice daily coal tar is £9 more costly over 1 year and only produces 0.00041 more QALYs than the least costly strategy mentioned above. This gives it an incremental cost-effectiveness ratio (ICER) of £22,658 which is just above the NICE £20,000 per QALY threshold.
The most effective strategy (once daily combined product containing calcipotriol monohydrate and betamethasone dipropionate then twice daily potent corticosteroid then twice daily coal tar) costs an additional £192 per year compared to the next most costly non-dominated strategy (concurrent steroid and vitamin D or vitamin D then twice daily potent steroid then twice daily coal tar), yet produces just 0.00107 additional QALYs for an ICER of over £179,000. Based on the results of this model, it appears that starting with once daily combined product containing calcipotriol monohydrate and betamethasone dipropionate, although most effective, is very unlikely to be cost-effective.
Results of the analysis showed that a strategy of using vehicle or emollient with no active agent only was the most costly and least effective, largely driven by the cost of referrals and specialist management for non-responders. Strategies that included once or twice daily vitamin D or vitamin D analogue were not cost-effective regardless of where they were included in the sequence. This is largely due to their relatively low rank in terms of effectiveness and their relatively high acquisition cost. Strategies that included dithranol were also all dominated, that is more costly and less effective than alternatives. Finally, strategies in which patients were referred after non-response to only 2 topicals were all dominated, thus not cost effective.
The probabilistic analysis indicates that there is a great deal of uncertainty as to which sequence is optimal (i.e. most cost effective). There appears to be very little difference between initial potent corticosteroid followed by concurrent potent corticosteroid and vitamin D or vitamin D analogue (one applied in the morning and one in the evening) and vice versa, with the difference in their net monetary benefits (NMB) being only £1 (£16,748 and £16,747 respectively) and both having an equal probability of being optimal at a £20,000 willingness to pay threshold. Generally, it looks as though a strategy of starting with either potent corticosteroids or concurrent treatment with potent corticosteroid and vitamin D or vitamin D analogue (one applied in the morning and one in the evening) is most likely to be cost-effective, whereas starting with once daily combined product containing calcipotriol monohydrate and betamethasone dipropionate is very unlikely to be cost-effective.
A series of sensitivity analyses suggested that the conclusions from the base case are sensitive to changes in some parameters and/or assumptions.
Sensitivity analyses – Treatment effects
The network meta-analysis of topical therapies was performed for two response outcomes: investigator assessed global improvement (IAGI) and patient assessed global improvement (PAGI). The economic evaluation used the investigator assessed outcome in the base case, largely because there was more data from the randomised evidence reported for this outcome. In a sensitivity analysis, treatment effects from the network meta-analysis of patient reported outcome was used.
Results of the analysis using patient reported outcomes indicates that starting treatment with once daily potent corticosteroids, moving on to the concurrent treatment if that fails and then trying twice daily vitamin D or vitamin D analogue is likely to be both the least costly and most cost-effective strategy given a threshold of £20,000 per QALY gained. Initial treatment with concurrent potent corticosteroid and vitamin D or vitamin D analogue (one applied in the morning and one in the evening) appears less cost-effective using patient reported outcomes than physician reported outcomes, unlikely to be cost-effective at thresholds less than £100,000. Once daily combined product containing calcipotriol monohydrate and betamethasone dipropionate, first or second line in a sequence, still looks to generate additional benefits (QALYs), but at additional costs unlikely to be considered good value for NHS resource (ICERs upwards of £115,000 per QALY gained).
The base case network meta-analysis of physician/investigator assessed response used in the base case cost-effectiveness analysis included all RCTs that met the inclusion criteria for the clinical review of direct evidence. The review of direct evidence was quite focused and as such did not include evidence for every possible pair wise comparison. In a sensitivity analysis of the network meta-analysis and thus the cost-effectiveness analysis, additional studies were included. For details on the particulars of these sensitivity analyses and what effect they had on the estimated treatment effects, see Appendix K.
When treatment effects were based on all relevant RCT data, the results of the base case changed only slightly. Twice daily potent corticosteroid followed by concurrent steroid and vitamin D or vitamin D analogue (one applied in the morning and one in the evening) is still likely to be optimal for first and second line treatments. However, instead of twice daily coal representing the optimal third line topical, twice daily vitamin D or vitamin D analogue looks to be most cost-effective. This sensitivity analysis calls into question whether vitamin D or vitamin D analogue or coal tar represents the better third line treatment option.
Sensitivity analysis – Variation in early versus late response
The base case assumed that patients would trial a given topical for up to 8 weeks and that some proportion of patients would be expected to respond by 4 weeks and discontinue treatment at that time. The remainder would carry on to 8 weeks, at which time non-responders would move on to the next topical in a sequence. The data defining the breakdown of early (at 4 weeks) vs late (at 8 weeks) responders was limited to two studies103,156 and GDG opinion and was thus very uncertain. Deterministic sensitivity analyses were performed around these parameters to observe the impact on the results.
First, an analysis was performed in which no one was expected to respond and discontinue treatment at 4 weeks (i.e. all responders require 8 weeks treatment). Compared to the results of the base case when all comparators are included, the rank order of strategies in terms of mean net benefits changed very little. The ICERs for strategies on the cost-effectiveness frontier (see ) increased relative to the base case, thus becoming less likely to be considered cost-effective. This analysis found that, given a NICE willingness-to-pay threshold of £20,000 per QALY gained, the most cost-effective strategy is likely to be one of starting with twice daily potent corticosteroid and moving to concurrent potent corticosteroid and vitamin D or vitamin D analogue (one applied in the morning and one in the evening) and then twice daily coal tar. This strategy was also the least costly strategy among the 118 modelled. Base case results for non-dominated and non-extendedly dominated strategies are presented .
Results showed that starting with concurrent potent corticosteroid and vitamin D or vitamin D analogue (one applied in the morning and one in the evening) and switching to twice daily potent corticosteroid and then twice daily coal tar is £9 more costly over 1 year and only produces 0.00041 more QALYs than the least costly strategy mentioned above. This gives it an incremental cost-effectiveness ratio (ICER) of £22,658 which is just above the NICE £20,000 per QALY threshold.
The most effective strategy (once daily combined product containing calcipotriol monohydrate and betamethasone dipropionate then twice daily potent corticosteroid then twice daily coal tar) costs an additional £192 per year compared to the next most costly non-dominated strategy (concurrent steroid and vitamin D or vitamin D then twice daily potent steroid then twice daily coal tar), yet produces just 0.00107 additional QALYs for an ICER of over £179,000. Based on the results of this model, it appears that starting with once daily combined product containing calcipotriol monohydrate and betamethasone dipropionate, although most effective, is very unlikely to be cost-effective.
Second, an analysis was performed in which all responders were assumed to respond by 4 weeks, with no one requiring an additional 4 weeks of treatment. The ICER for all strategies on the cost-effectiveness plane (see ) decreased relative to the base case, and now starting with concurrent therapy and moving to twice daily potent corticosteroids looks to be cost-effective at a £20,000 threshold compared to potent corticosteroids and then concurrent therapy. Initial treatment with once daily TCF product is still unlikely to be cost-effective, with an ICER of more than £140,000.
Finally, an analysis was performed in which a 4-week stopping rule was applied. In this scenario, responders were limited to those that have responded by week 4, and all other patients are assumed to move on to the next topical in the sequence (i.e. no one continues to 8 weeks of treatment with the same topical). Relative to the base case, the total costs for all strategies more than doubled as more patients were classified as non-responders and moved down the care pathway reaching referral to secondary care. Starting with concurrent therapy and then moving to twice daily potent corticosteroids was now the least costly strategy and most likely to be cost-effective. The ICER for once daily TCF product instead of concurrent therapy in this sequence decreased substantially relative to the base case (£174,000 to £94,000) but is still unlikely to be considered cost-effective at the NICE threshold.
Sensitivity analysis – Reduced adherence
There was some concern that issues of treatment adherence were inadequately captured in the model. The estimates of effect used in the base case were derived from randomised controlled trials which may represent the best case scenario for topical therapies. The GDG wished to explore how reduced adherence to twice daily treatments would affect the conclusions of the base case. In this scenario, 60% of patients being treated with twice daily topical were assumed to adhere to twice daily treatment whilst the remaining 40% of patients were assumed to apply the topical only once daily. For concurrent therapy, the 40% were assumed to adhere to once daily potent corticosteroid treatment only. Efficacy of the twice daily treatments would thus be reduced compared to the base case estimates. To be conservative, no reductions in cost were assumed despite the fact that less topical would be used.
With adherence reduced, there is no change substantive change to the results of the base case. Total costs across all strategies increase slightly (average of £27 more) and benefits decreased very slightly (average of 0.0007 fewer QALYs), but the conclusions from the base case remain unchanged. The most cost-effective strategy, given a £20,000 per additional QALY threshold is still twice daily potent corticosteroid followed by concurrent therapy and then twice daily coal tar. To put concurrent therapy before twice daily potent corticosteroids has an ICER of £36,000 (up from £23,000 in base case) and to replace concurrent therapy with once daily TCF before steroids has an ICER of £76,609 (down from £174,545 in the base case).
Sensitivity analysis – Utility values
In the base case, the mean utility gain associated with achieving some level of improvement, but not clearance or near clearance was assumed to be 0.05. This value was based on a downward adjustment of a value used in a recent cost-utility analysis included in the health economic review. Bottomley and colleagues37 modelled a utility gain of 0.07 for non-responders compared to baseline. To see what effect the GDG adjustment had on the results, the Bottomley figure (0.07) was used in a sensitivity analysis
Results indicate that the conclusion about cost-effectiveness changes very little using this more optimistic estimate of utility gain. The ICERs for all strategies increases relative to the base case; therefore, starting with concurrent treatment before twice daily potent corticosteroids is less likely to be cost-effective (ICER=£88,333 vs £23,250 in the base case). Similarly, the ICER for a strategy starting with combined product containing calcipotriol monohydrate and betamethasone dipropionate increased to over £787,000 compared to starting with concurrent treatment (£174,500 in the base case).
Sensitivity analysis – 4-week quantity of combined product containing calcipotriol monohydrate and betamethasone dipropionate
In the base case, hypothetical patients are assumed to use 134.0 g of combined product containing calcipotriol monohydrate and betamethasone dipropionate during 4 weeks of treatment. Bottomley and colleagues used a much lower value for this input (92.6 g), and we explored how the results of the NCGC analysis might change if this lower estimate was used. The cost of 92.6 g of combined product containing calcipotriol monohydrate and betamethasone dipropionate was £61.27 (compared to £94.26 in the base case). The results of this sensitivity analysis showed that the ICER for combined product containing calcipotriol monohydrate and betamethasone dipropionate improved compared to the base case (£124,400 vs £174,545); however this is still well above the NICE cost-effectiveness threshold of £20,000 per additional QALY. Initial therapy with twice daily potent corticosteroid or concurrent vitamin D or vitamin D analogue and potent corticosteroid (one applied in the morning and one in the evening) is still more likely to be considered cost-effective.
Sensitivity analysis – Unit cost of potent corticosteroids and vitamin D and vitamin D analogues
The base case assumed that the cost for each topical was based on the product and formulation with the lowest unit cost per gram/millilitre. Given that clinicians and patients may have preferences for different products or formulations, it was considered necessary to explore how variation in price of topicals, particularly potent corticosteroids and vitamin D, might affect the results. To do this, the highest cost (per gram) potent corticosteroid Synalar gel (fluocinolone acetonide) was assumed in place of Betnovate cream or ointment. The cost of Synalar gel is around four times that of Betnovate cream/ointment. In another analysis, the most costly vitamin D ointment, Curatoderm (tacalcitol), was assumed instead of Silkis (calcitriol). The cost of Curatoderm is around 2.5 times more costly than Silkis and 1.6 times more costly than Dovonex (calcipotriol) ointment. In a final sensitivity analysis, both Synalar gel and Curatoderm were used.
Sensitivity analyses – Restricted comparators
The base case analysis put a several conditions on the way topicals could be sequenced (see in section 8.1.19.3). These conditions did not restrict how potent corticosteroids were fit into treatment sequences other than that they could not appear in all three lines of treatment. This included their use as part of concurrent or combined treatment. The GDG expressed concern that these restrictions may not fully reflect the caution they would use in prescribing trials of potent corticosteroids, in that the BNF discourages continuous use of potent corticosteroids for more than 8 weeks at a time. The GDG was also concerned that the analysis did not fully capture the safety risks associated with the continuous or intermittent use of twice daily potent steroids. In a series of sensitivity analyses, various additional restrictions were placed on the treatment sequences.
In the first scenario, it was assumed that interventions that included potent corticosteroids could not be offered consecutively. For example, once daily combined product containing calcipotriol monohydrate and betamethasone dipropionate could not be offered after treatment with once or twice daily potent corticosteroids, nor could twice daily potent corticosteroid follow once daily potent corticosteroid. Under this assumption, starting with twice daily corticosteroid, then trying twice daily vitamin D or vitamin D analogue and then using both potent corticosteroid and vitamin D or vitamin D analogue concurrently (one applied in the morning and one in the evening) would represent the best value for NHS resources given a £20,000 per QALY threshold. Starting with concurrent treatment would only be cost-effective at thresholds of greater than £33,000 and combined product containing calcipotriol monohydrate and betamethasone dipropionate would only be cost-effective at thresholds over £202,000.
In the second scenario, it was assumed that twice daily corticosteroid could not be prescribed as a first or second line topical therapy, but consecutive use of potent corticosteroids was permitted. Under this scenario, the optimal strategy was to start with concurrent corticosteroid and vitamin D or vitamin D analogue (one applied in the morning and one in the evening), then try twice daily vitamin D or vitamin D analogue alone and finally twice daily potent corticosteroid only. This had an ICER of £18,000 per QALY gained compared to once daily potent corticosteroid followed by concurrent treatment and then twice daily coal tar. Strategies including combined product containing calcipotriol monohydrate and betamethasone dipropionate either as second or first line were not cost-effective unless the threshold was over £110,000 and £446,000, respectively.
A third scenario combined the first and second scenarios, such that twice daily potent corticosteroid could not be prescribed as first or second line treatment and no sequences could include consecutive lines of potent steroid containing strategies. Under these conditions, the same sequence as in scenario 2 is most cost-effective (concurrent – vit D BD – PS BD). Combined product containing calcipotriol monohydrate and betamethasone dipropionate replaces twice daily steroid in that sequence only if the threshold willingness to pay is £134,000 and replaces concurrent treatment in the same sequence if the threshold is £202,000.
In a fourth and final scenario, twice daily potent corticosteroid was removed entirely and no potent steroid containing products could be prescribed consecutively. Under this assumption, the most cost-effective sequence was initial concurrent treatment followed by twice daily vitamin D or vitamin D analogue alone and then twice daily coal tar. Combined product containing calcipotriol monohydrate and betamethasone dipropionate replaces twice daily coal tar in that sequence at a threshold of over £47,000 and replaces concurrent treatment at a threshold of over £489,000.
Sensitivity analyses – downstream resource use and cost
Changes to the assumed probability of referral to secondary care and proportion offered phototherapy have no meaningful effect on the conclusions of the base case. The probability of referral to secondary care was varied downwards to 40% and upward to 80%. When referral occurred less often than in the base case, there was no change to the rank order of strategies, but the ICER for a strategy where combined product containing calcipotriol monohydrate and betamethasone dipropionate was used first instead of concurrent treatment increased to £200,000 per additional QALY. When referral occurred more often than in the base case, there was still no change in the rank order, but the ICER for combined product containing calcipotriol monohydrate and betamethasone dipropionate was slightly lower. If the probability of undergoing UVB phototherapy upon referral was higher than in the base case (50% vs 30%), then the ICER for combined product containing calcipotriol monohydrate and betamethasone dipropionate compared to concurrent treatment reduced slightly, but not enough to make it cost-effective. Finally, if instead of assuming patients are treated with UVB phototherapy, it is assumed they receive outpatient day care treatment with specialist supervised topical therapies, then the ICER for concurrent therapy before potent corticosteroids alone increases to over £30,000 per QALY and the ICER for initial combined product containing calcipotriol monohydrate and betamethasone dipropionate instead of concurrent therapy decreases to £155,000 per QALY.
If the time horizon is extended for 2 to 3 years and cumulatively more patients see a specialist and move on to UVB phototherapy, then initial treatment with concurrent vitamin D or vitamin D analogue and potent corticosteroids (one applied in the morning and one in the evening) becomes more cost-effective than starting with potent corticosteroids alone. When the time horizon is extended, TCF product becomes more cost-effective compared to concurrent treatment (ICER = £118,067 at 2 years; ICER = £90,710 at 3 years; ICER=£75,255 at 5 years; ICER=£73,541 at 10 years), but is still very unlikely to be considered cost effective given the NICE willingness to pay threshold of £20,000 per QALY gained. Visual inspection of the health state membership probabilities over a 10-year time horizon indicates that patients are no longer transitioning between health states after 8 years because they have all reached long-term management with a GP or specialist by this point. This suggests that the ICER for TCF product is unlikely to come down any further even if the model time horizon is extended beyond 10 years.
8.1.19.5. Interpretation and limitations
In assessing the relative cost-effectiveness of alternative topical therapies in patients with mild to moderate psoriasis limited evidence was available from the published economic literature. The evidence that was identified and included in the health economic review had potentially serious limitations and therefore the GDG considered it a priority to undertake original evaluation for the guideline in order to inform recommendations. This analysis showed that there were relatively small differences in terms of benefit between different topical sequences, but the differences in terms of cost were quite substantial. Based on the mean costs and benefits, the analysis suggests that initial treatment with potent corticosteroids followed by concurrent treatment with potent corticosteroid and vitamin D or vitamin D analogue (one applied in the morning and one in the evening) and followed then by twice daily coal tar therapy is likely to represent the most cost-effective sequence for implementation in primary care. Uncertainties in the analysis were explored through sensitivity analysis which showed that in some scenarios
Once daily potent corticosteroid or concurrent treatment should come first in the sequence
Twice daily vitamin D or vitamin D analogue should come second or third in the sequence, after concurrent treatment
Combined product containing calcipotriol monohydrate and betamethasone dipropionate should be offered third in the sequence, after potent corticosteroids and concurrent treatment
Sequences starting with once daily combined product containing calcipotriol monohydrate and betamethasone dipropionate were slightly more effective than the same sequence starting with concurrent potent corticosteroid and vitamin D or vitamin D analogue (one applied in the morning and one in the evening); however, the very modest additional benefit (0.0011) would only be considered potentially cost-effective if willingness to pay thresholds were between £ 100,000 and £ 500,000 per QALY gained.
The analysis has several limitations which were considered carefully by the GDG. Firstly, the analysis evaluates treatment sequences even though the available trial data compares single topicals head to head without sequencing. In order to apply the treatment effects within the sequencing model, we assumed that treatment effects were independent. That is, we assumed the effectiveness of combined product containing calcipotriol monohydrate and betamethasone dipropionate as a second or third line topical was equal to its effectiveness as a first line agent and that this was true regardless of other topicals it may follow. The GDG did not believe this to be a significant limitation given that the patients included in the overwhelming majority of RCTs were reported to have psoriasis for longer than 5 years, during which the can be assumed to have previously tried, succeeded and/or failed various topical treatments.
The analysis only captured the efficacy of topicals and did not capture the costs or consequences of adverse events. Although the RCT evidence on adverse events was sparse, the GDG is conscious of the risks associated with the long-term use of potent and very potent corticosteroids. They carefully considered whether the added effect in terms of clearance was worth the potential risks of adverse effects.
The model was also focused on the induction of disease clearance as opposed to the maintenance of clearance. Trials focusing on maintenance were limited in number and inadequately reported for use in the economic model. In particular, there was uncertainty as to how maintenance treatments were applied in the trials and therefore incorporating such evidence and assumptions into the model was considered too difficult and unlikely to be valid.
The model also takes a relatively short time horizon considering that psoriasis is a chronic, long term condition for which patients may undergo treatment for many years of their lives. Frequency and severity of relapse, selection for and speed of onward referral, methods of self-management and long-term safety are all issues inadequately addressed in the evidence base and therefore translate into limitations of the economic analysis.
The model estimated the health gain for each treatment by mapping the change in PASI score to the EQ-5D based on observational evidence. However, it has been noted that several important areas of health-related quality of life for people with psoriasis are not directly assessed by the EQ-5D questionnaire226. Therefore it is possible that the EQ-5D may lack content validity for these patients. Research is ongoing in this area. But we note that even using a £ 30,000 per QALY threshold rather than £ 20,000 would not change the conclusions of our analyses. Therefore only if the EQ-5D is under-estimating health gain of one treatment compared to another by a considerable extent, could this pose a serious limitation.
8.1.19.6. Comparison with published studies
The findings from the NCGC original economic analysis are quite different from the results of the most similar published study by Bottomley and colleagues37. Bottomley and colleagues found 8 weeks of once daily combined product containing calcipotriol monohydrate and betamethasone dipropionate to dominate other modelled strategies including once and twice daily vitamin D or vitamin D analogue followed by potent corticosteroid, potent corticosteroid followed by vitamin D or vitamin D analogue and 8 weeks of concurrent treatment with vitamin D or vitamin D analogue and potent corticosteroid (one applied in the morning and one in the evening). Although the analysis appears to have been executed well, the estimates of effect and resource use had limitations which called the conclusions of the analysis into question.
The biggest differences in the results of the NCGC analysis presented here and the analysis undertaken by Bottomley has to do with the treatment effect sizes used. In their analysis, concurrent treatment was found to be very ineffective, with just 14.9% of patients responding with a PASI75 compared to the combined product containing calcipotriol monohydrate and betamethasone dipropionate to which 50.3% of patients responded (RR=3.38). The NCGC analysis showed a much small difference between these treatments, with 65.1% of patients responding to concurrent treatment and 70.7% responding to The combined product containing calcipotriol monohydrate and betamethasone dipropionate (RR=1.09).
In addition, the estimate they used for quantity of topical used per 4-week treatment period was 92.6 g, compared to the estimate used in the NCGC analysis 134.0 g. Based on these estimates of resource use, the NCGC analysis assumes 4 weeks of the combined product containing calcipotriol monohydrate and betamethasone dipropionate costs £ 29.26 more than Bottomley and colleagues did. Furthermore, the difference between the combined product containing calcipotriol monohydrate and betamethasone dipropionate and concurrent treatment is different between the analyses. The additional cost of the combined product containing calcipotriol monohydrate and betamethasone dipropionate was £ 36.91 in Bottomley and more than twice that, £ 76.34, in the NCGC analysis. We performed a sensitivity analysis in which we assumed the same quantity of the combined product containing calcipotriol monohydrate and betamethasone dipropionate used by Bottomley and colleagues (i.e. 92.6 g, £ 61.27). The ICER for the combined product containing calcipotriol monohydrate and betamethasone dipropionate improved compared to the base case (£ 124,400 vs £ 174,545), but was still well above the NICE cost-effectiveness threshold of £ 20,000 per additional QALY.
The one thing that Bottomley and colleagues were able to capture that the NCGC analysis was not had to do with the potential disutilities associated with adverse events; however these inputs were not reported, were not included in their base case and, their impact on the results were not reported in full. The authors simply state that the influence of AEs ‘had no impact on the results.’