NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
1. Fluid therapy for the management of diabetic ketoacidosis
1.1. Review question
In children and young people with diabetic ketoacidosis:
- What is the appropriate route of fluid administration for rehydration?
- What fluids (including additives) should be used for rehydration?
- At what rate, including volume of fluid should children and young people be rehydrated?
1.1.1. Introduction
Diabetic ketoacidosis (DKA) is a life-threatening condition that can occur in children and young people with type 1 diabetes. It can also affect some children and young people with type 2 diabetes. Management of DKA involves the replacement of fluids and electrolytes. The 2015 NICE guidance on the diabetes (type 1 and type 2) in children and young people: diagnosis and management included recommendations on fluid therapy that covered the route of administration, types of fluids and additives that should be given as well as the volume and rate of fluid administration.
The topic was reviewed by NICE’s surveillance team and new evidence was identified which prompted a partial update of the guideline. The aim of this review is to determine the optimal route of administration, type of fluid (including additives) and rate and volume for rehydration in children and young people with DKA.
1.1.2. Summary of the protocol
| PICO Table | |
|---|---|
| Population | Children and young people with type 1 or type 2 diabetes with diabetic ketoacidosis (although the diabetes may not yet have been recognised, for example, if the child or young person is presenting for the first time with DKA) |
| Intervention |
Route of administration:
Oral:
|
| Comparator | Route of administration:
|
| Outcomes |
|
1.1.3. Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Methods specific to this review question are described in the review protocol in appendix A and appendix B.
Four studies were identified that focused on children and young people with type 1 diabetes while the remaining studies did not specify type of diabetes. The committee highlighted that the management of DKA in children with type 1 or type 2 diabetes does not differ. Therefore, these studies were not downgraded for indirectness.
Additionally, some studies included children and young people with severe to moderate DKA whilst the majority of studies included children with all severities of DKA. Therefore, evidence has been presented by severity of DKA.
Declarations of interest were recorded according to NICE’s conflicts of interest policy (2018).
1.1.4. Effectiveness evidence
1.1.4.1. Included studies
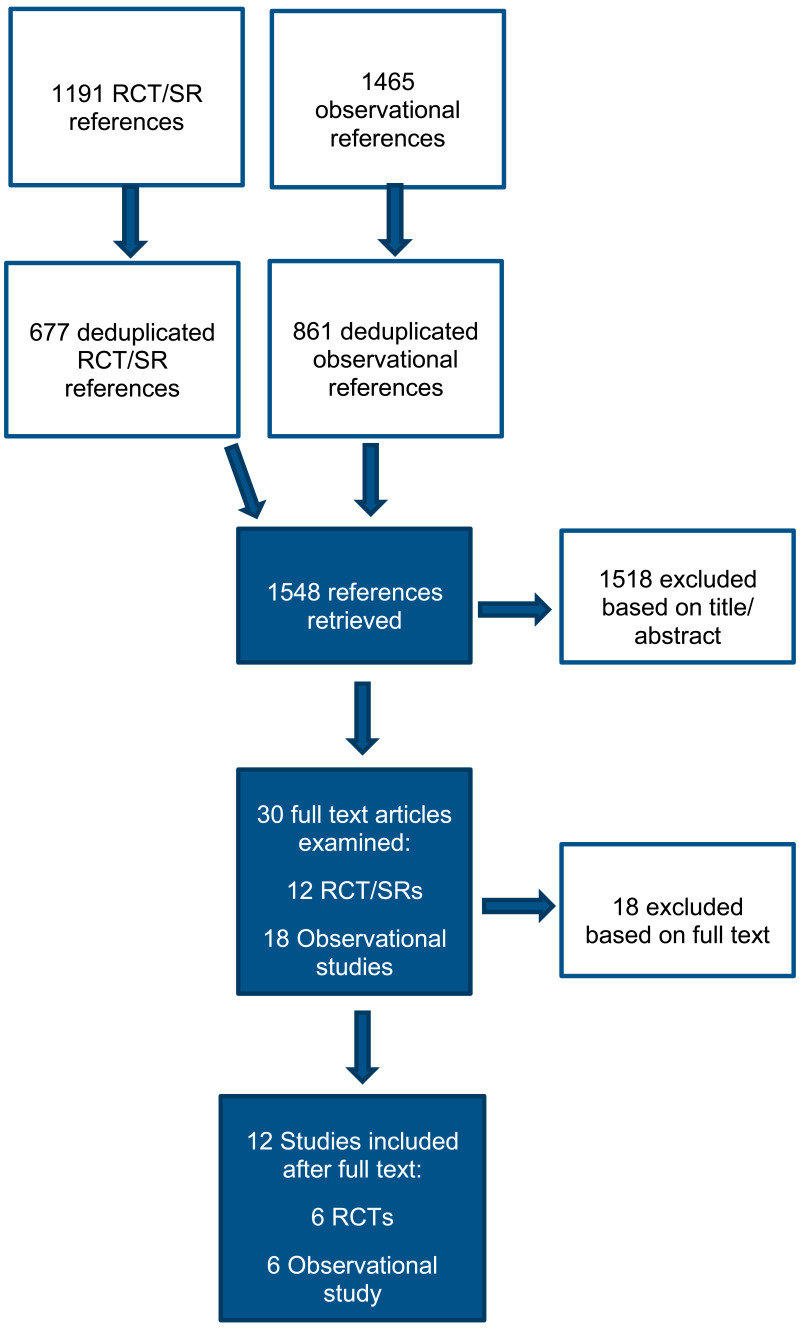
A total of 1,191 RCTs and systematic reviews and 1,456 observational studies were identified in the search. After removing duplicate references, 677 RCTs and systematic reviews and 861 observational studies were screened at title and abstract stage. 10 additional studies (1 RCT and 9 observational studies) were identified from the 2015 NICE guidance on diabetes (type 1 and type 2) in children and young people: diagnosis and management. Overall, a total of 1,548 studies were screened.
Following title and abstract screening, 30 studies (12 RCTs and systematic reviews and 18 observational studies) were included for full text screening. These studies were reviewed against the inclusion criteria as described in the review protocol (Appendix A). Overall, 12 studies were included (6 RCTs and 6 retrospective cohort studies).
Route of administration
Studies which compared route of administration were not identified.
Type of fluids – oral fluids
Studies which compared different oral fluids were not identified.
Type of fluids – IV fluids
7 studies (4 RCTs, and 3 retrospective cohort studies) were identified which examined the type of fluid for rehydration:
- 3 studies examined the type of fluid that should be used as the initial IV fluid in children and young people with DKA. The following IV fluids were examined:
- 0.9% saline vs Hartmann’s solution
- 0.9% saline vs Plasma-Lyte-A
- 0.9% saline vs hypertonic saline (3% NaCl)
- 1 study examined the type of fluid that should be used for the replacement of deficit in children and young people with DKA. The following IV fluids were examined:
- 0.9% saline vs 0.45% saline
- 1 study examined the type of fluid that should be used as post bolus rehydration fluid in children and young people with DKA. The following IV fluids were examined:
- 0.9% saline vs 0.45% saline
- 1 study examined the type of fluid that should be used as after initial rehydration in children and young people with DKA. The following IV fluids were examined:
- 74 mEq/L NaCl vs 100 mEq/l of NaCl
- 1 retrospective cohort study compared the use of normal saline vs Ringer’s lactate in children and young people with DKA.
IV fluids + additives
- 1 retrospective cohort study compared the use of IV fluid (lactate Ringers or lactate Ringers with saline) and sodium carbonate with IV fluid alone.
- 1 retrospective cohort study compared the use of IV fluids (not defined) with sodium bicarbonate with no sodium bicarbonate.
Rate of rehydration
3 studies (2 RCTs, and 1 retrospective cohort study) were identified which examined the rate of rehydration:
- 1 RCT compared fast rate with slow rate of rehydration in children and young people with DKA
- 1 RCT compared rapid rate with slower rate of rehydration in children and young people with T1DM presenting with DKA
- 1 retrospective study compared fast rate with slow rate of rehydration in children and young people with T1DM presenting with DKA
Volume of fluid
- 1 RCT compared high volume of IV fluid with low volume of IV for rehydration in children and young people with T1DM presenting with DKA.
An MHRA search was also conducted. However, no recent drug safety alerts or recalls were identified.
See appendix E for evidence tables and reference section.
1.1.4.2. Excluded studies
Overall, 18 studies were excluded. See appendix L for list of excluded studies.
1.1.5. Summary of studies included in the effectiveness delivery evidence
Type of fluids - IV fluids
| Reference | Study type | Population | Intervention | Comparator | Outcomes | Further notes |
|---|---|---|---|---|---|---|
| Basnet 2014 | Retrospective cohort study | Children between the age of 1 and 18 years with initial serum pH <7.3 and serum bicarbonate <15 meq/L with hyperglycaemia and ketonuria |
0.9% saline Used a post-bolus re-hydration fluid during the recovery phase of DKA |
0.45% saline Used a post-bolus re-hydration fluid during the recovery phase of DKA |
|
|
| Bergmann 2018 | Retrospective cohort study | Children aged 0 to 17 years discharged from inpatient, observation, or emergency department (ED) care with a diagnosis of diabetes with ketoacidosis, type I (International Classification of Diseases, Ninth Revision [ICD-9] codes 250.11 and 250.13), between January 1, 2005, and September 30, 2015 |
Normal saline No information provided on DKA protocols used. |
Ringer’s lactate No information provided on DKA protocols used. |
|
|
| Kupperman 2018 | RCT | Children aged between 0 and 18 years of age and had a diagnosis of diabetic ketoacidosis |
0.45% sodium chloride solution Standard initial bolus: 10 ml per kilogram bolus of 0.9% sodium chloride solution. Additional intravenous fluid bolus:
|
0.9% sodium chloride solution Standard initial bolus: 10 ml per kilogram bolus of 0.9% sodium chloride solution. Additional intravenous fluid bolus:
|
|
|
| Savaş-Erdeve 2011 | Retrospective cohort study | Patients younger than 18 years of age who were admitted to the paediatric intensive care unit from 2002 to 2009 |
75 mEq/L Sodium Chloride Initial rehydration was performed with isotonic solutions in the first hour of treatment. The total volume to be given was calculated assuming a 10% deficit plus maintenance fluid. Amounts of fluids used in the initial resuscitation were subtracted from the total volume calculated for 48 hours and the infusion rate was adjusted accordingly. The patients in Group I had received IV fluids with a Na concentration of 75 mEq/L (1/2 isotonic NaCl plus 1/2 5% dextrose). |
100 mEq/L Sodium Chloride Initial rehydration was performed with isotonic solutions in the first hour of treatment. The total volume to be given was calculated assuming a 10% deficit plus maintenance fluid. Amounts of fluids used in the initial resuscitation were subtracted from the total volume calculated for 48 hours and the infusion rate was adjusted accordingly. The patients in Group II had received IV fluids with a Na concentration of 100 mEq/L (2/3 isotonic NaCl plus 1/3 5% dextrose). |
|
|
| Shafi 2018 | RCT | Subjects with age ≤18 years with a diagnosis of DKA were screened for the inclusion in the study and were included if they met the criteria for having moderate-severe DKA |
0.9% normal saline Children randomised to the 0.9% saline received 20 ml/kg of solution during the initial 1 hour of fluid therapy. The rest of the fluid and management was per the written DKA management protocol followed by the treating unit, which is based on the ISPAD clinical practice consensus guidelines. |
Hypertonic Saline (3% NaCl) Children randomised to the hypertonic saline (3% NaCl) received 20 ml/kg of solution during the initial 1 hour of fluid therapy. The rest of the fluid and management was per the written DKA management protocol followed by the treating unit, which is based on the ISPAD clinical practice consensus guidelines. |
|
|
| Williams 2020 | RCT | All consecutive children > 1 month to < 12 years who presented to the paediatric emergency room with DKA as defined by the International Society of Paediatric and Adolescent Diabetes (ISPAD-2014) were enrolled into the study |
0.9% normal saline Volume calculated based on deficit (6.5-10%) and maintenance fluid as per Holliday Segar. Fluids given over 48 hours as hourly infusion. Eligible children who presented in shock [perfusion abnormalities with or without hypotension (blood pressure < 5th centile for age)], received trial fluid bolus of 20 ml/kg over an hour. |
Plasma-Lyte-A Volume calculated based on deficit (6.5-10%) and maintenance fluid as per Holliday Segar. Fluids given over 48 hours as hourly infusion. Eligible children who presented in shock [perfusion abnormalities with or without hypotension (blood pressure < 5th centile for age)], received trial fluid bolus of 20 ml/kg over an hour. |
|
|
| Yung 2017 | RCT | Children with moderate to severe DKA admitted to the paediatric intensive care unit (PICU) or high-dependency unit with DKA were eligible. |
Hartmann’s solution After resuscitation, subjects were randomised to Hartmann’s solution as their initial fluid for at least 12 hours. |
0.9% normal saline After resuscitation, subjects were randomised to 0.9% normal saline as their initial fluid for at least 12 hours. |
|
|
IV fluids + Additives
| Reference | Study type | Population | Intervention | Comparator | Outcomes | Further notes |
|---|---|---|---|---|---|---|
| Green 1998 | Retrospective cohort study | Children aged 15 years or younger with a hospital diagnosis of severe DKA |
Sodium bicarbonate Children received standard DKA therapy with hydration and intravenous insulin infusion. Adjunctive bicarbonate therapy was administered by treating physicians in doses ranging from 7 to 238 mEq and from 0.53 to 7.37 mEq/kg (mean 2.08, median 1.66 mEq |
No sodium bicarbonate Children received standard DKA therapy with hydration and intravenous insulin infusion. |
|
|
| Mar 1981 | Retrospective cohort study | Children with diabetes with DKA with at least one episode of DKA |
Sodium bicarbonate and saline and lactate Ringers or sodium bicarbonate and Lactate Ringers IV solution with sodium bicarbonate No information about DKA protocol provided. |
Lactate Ringers or Lactate Ringers with saline No sodium bicarbonate No information about DKA protocol provided. |
|
|
Rate of rehydration
| Reference | Study type | Population | Intervention | Comparator | Outcomes | Further notes |
|---|---|---|---|---|---|---|
| Felner 2001 | Retrospective cohort study | Patients within insulin-dependent diabetes mellitus who received DKA therapy under a traditional fluid protocol (group 1)were identified from a list of patients at Children’s Medical Centre of Dallas who has discharge diagnoses of ‘diabetic ketosis/ketoacidosis” and admission dates from September 1st 1994 to June 30th 1997, whereas patients treated under the revised fluid protocol (group 2) were identified from a list of patients admitted from July 1st 1997 to March 31st 2000. |
Fast rate The fluid deficit was calculated by multiplying the percentage of dehydration (7-10%, determined clinically on initial presentation) by the patient’s weight. The fluid deficit was added to 1.5 times the patient’s total fluid requirement. Half of the total required fluid was ordered over the first 12 hours of treatment and the remaining 50% over the next 24 hours. |
Slow rate Total fluids were delivered at 2.5 times the maintenance rate regardless of the degree of dehydration. Fluid were decreased to 1 to 1.5 times the maintenance rate after 24 hours of treatment (or earlier if acidosis resolved) until urine ketones were negative. |
|
|
| Glaser 2013 | RCT | Children aged 8 to 18 years old, were diagnosed with type 1 diabetes and had DKA |
Rapid rate Intravenous fluid bolus: 20 mL/Kg Assumed fluid deficit: 10% of body weight Rate of deficit replacement: Two-thirds over first 24 h; One-third over next 24 h Urine output replacement: Half of urine vol replaced while serum glucose level is >250 mg/dL Fluid type: 0.9% saline while serum glucose is >250 mg/dL, followed by 0.45% saline. |
Slower rate Intravenous fluid bolus: 10 mL/Kg Assumed fluid deficit: 7% of body weight Rate of deficit replacement: Evenly over 48 h Urine output replacement: None Fluid type: 0.9% saline while serum glucose is >250 mg/dL, followed by 0.45% saline. |
|
|
| Kuppermann 2018 | RCT | Children aged between 0 and 18 years of age and had a diagnosis of diabetic ketoacidosis |
Fast administration of sodium chloride Process of replacement of deficit: During the initial 12 hours, replace half the fluid deficit, plus maintenance fluids. Then replace remaining deficit, plus maintenance fluids, during the subsequent 24 hours. Fluid used for replacement of deficit: 0.45% and 0.9% sodium chloride (data available separately for different solution) |
Slow administration of sodium chloride Process of replacement of deficit: Replace deficit, plus maintenance fluids, evenly during a period of 48 hours. Fluid used for replacement of deficit: 0.45% and 0.9% sodium chloride (data available separately for different solution) |
|
|
Volume of rehydration
| Reference | Study type | Population | Intervention | Comparator | Outcomes | Further notes |
|---|---|---|---|---|---|---|
| Bakes 2016 | RCT | Children were eligible for participation if they were between 0 and 18 years of age, had type 1 diabetes mellitus plus the presence of DKA |
High volume IV fluid The high-volume IV fluid group, received a 20 mL/kg of IV 0.9% saline bolus over the first hour followed by 0.675% saline + potassium replacement at 1.5 times maintenance. |
Low volume IV fluid Low-volume IV fluid group, received a 10 mL/kg of IV 0.9% saline bolus over the first hour followed by 0.675% saline + potassium replacement at 1.25 times maintenance. |
|
|
See appendix E for full evidence reviews.
1.1.6. Summary of the effectiveness evidence
Type of fluid - IV fluids
Moderate to severe DKA
0.9% Saline vs Hartmann’s solution as initial IV fluid
Outcomes during treatment of DKA
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Minimum sodium concentration – MD greater than 1 favours 0.9% saline | |||||
| Yung 2017 | RCT | 77 | MD: 0.00 (−1.47, 1.47) | High | Could not differentiate between IV fluids |
| Maximum chloride concentration – MD greater than 1 favours 0.9% saline | |||||
| Yung 2017 | RCT | 77 | MD: 2.00 (−0.27, 4.27) | Moderate | Could not differentiate between IV fluids |
| Altered conscious state (defined as deterioration in Glasgow Coma Scale (CGS))– RR less than 1 favours 0.9% saline | |||||
| Yung 2017 | RCT | 77 | RR: 2.92 (0.12, 69.64) | Low | Could not differentiate between IV fluids |
| Acute renal failure - RR less than 1 favours 0.9% saline | |||||
| Yung 2017 | RCT | 77 | RR: 2.92 (0.12, 69.64) | Moderate | Could not differentiate between IV fluids |
0.9% Saline vs hypertonic saline (3% NaCl) as initial IV fluid
Outcomes during 1 hour of treatment
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Chloride concentration (mEq/L) - MD less than 1 favours 0.9% saline | |||||
| Shafi 2018 | RCT | 40 | MD −5.70 (−9.81, −1.59) | Low | 0.9% saline favoured |
Outcomes during 12 hours of treatment
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Cerebral oedema - RR less than 1 favours 0.9% saline | |||||
| Shafi 2018 | RCT | 40 | RR: 1.00 (0.07, 14.90) | Low | Could not differentiate between IV fluids |
All severities of DKA
0.9% Saline vs Plasma-Lyte A as initial IV fluid
Outcomes during 24 hours of treatment
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Incidence of acute kidney injury (AKI) (defined with either KDIGO or pRIFLE criteria)– RR less than 1 favours 0.9% saline | |||||
| Williams 2020 | RCT | 66 | RR: 0.80 (0.19, 3.29) | Low | Could not differentiate between IV fluids |
Outcomes during 48 hours of treatment
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Incidence of acute kidney injury (AKI) (defined with either KDIGO or pRIFLE criteria)– RR less than 1 favours 0.9% saline | |||||
| Williams 2020 | RCT | 66 | RR: 0.35 (0.04, 3.23) | Low | Could not differentiate between IV fluids |
Outcomes till discharge
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Healthcare utilisation – Need for renal replacement therapy - RR less than 1 favours 0.9% saline | |||||
| Williams 2020 | RCT | 66 | RR: 0.21 (0.01, 4.26) | Low | Could not differentiate between IV fluids |
| Healthcare utilisation – Need for ventilation - RR less than 1 favours 0.9% saline | |||||
| Williams 2020 | RCT | 66 | RR: 0.53 (0.05, 5.58) | Low | Could not differentiate between IV fluids |
| Mortality in hospital - RR less than 1 favours 0.9% saline | |||||
| Williams 2020 | RCT | 66 | RR: 0.21 (0.01, 4.26) | Low | Could not differentiate between IV fluids |
| Cerebral oedema - RR less than 1 favours 0.9% saline | |||||
| Williams 2020 | RCT | 66 | RR: 0.35 (0.01, 8.38) | Very low | Could not differentiate between IV fluids |
0.9% Saline vs. 0.45% saline for replacement of deficit
Outcomes during treatment of DKA
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours 0.9% saline | |||||
| Kuppermann 2018 | RCT | 1361 | RR: 1.27 (0.72, 2.22) | Moderate | Could not differentiate between IV fluids |
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours 0.9% saline – fast rate | |||||
| Kuppermann 2018 | RCT | 682 | RR: 1.07 (0.46, 2.50) | Moderate | Could not differentiate between IV fluids |
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours 0.9% saline– slow rate | |||||
| Kuppermann 2018 | RCT | 679 | RR: 1.44 (0.68, 3.06) | Moderate | Could not differentiate between IV fluids |
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours 0.9% saline - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0)) | |||||
| Kuppermann 2018 | RCT | 282 | RR: 1.66 (0.81, 3.38) | Moderate | Could not differentiate between IV fluids |
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours 0.9% saline - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0))- fast rate | |||||
| Kuppermann 2018 | RCT | 131 | RR: 1.62 (0.50, 5.27) | Moderate | Could not differentiate between IV fluids |
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours 0.9% saline - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0))- slow rate | |||||
| Kuppermann 2018 | RCT | 151 | RR: 1.68 (0.69, 4.10) | Moderate | Could not differentiate between IV fluids |
| Clinically apparent brain injury - RR less than 1 favours 0.9% saline | |||||
| Kuppermann 2018 | RCT | 1389 | RR: 0.70 (0.22, 2.21) | Low | Could not differentiate between IV fluids |
| Clinically apparent brain injury - RR less than 1 favours 0.9% saline – fast rate | |||||
| Kuppermann 2018 | RCT | 695 | RR: 0.98 (0.14, 6.92) | Low | Could not differentiate between IV fluids |
| Clinically apparent brain injury - RR less than 1 favours 0.9% saline – slow rate | |||||
| Kuppermann 2018 | RCT | 694 | RR: 0.59 (0.14, 2.46) | Low | Could not differentiate between IV fluids |
| Clinically apparent brain injury - RR less than 1 favours 0.9% saline - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0)) | |||||
| Kuppermann 2018 | RCT | 303 | RR: 1.03 (0.26, 4.02) | Low | Could not differentiate between IV fluids |
| Clinically apparent brain injury - RR less than 1 favours 0.9% saline - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0))- fast rate | |||||
| Kuppermann 2018 | RCT | 141 | RR: 0.96 (0.06, 15.02) | Low | Could not differentiate between IV fluids |
| Clinically apparent brain injury - RR less than 1 favours 0.9% saline - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0))- slow rate | |||||
| Kuppermann 2018 | RCT | 162 | RR: 1.05 (0.22, 5.05) | Low | Could not differentiate between IV fluids |
| Mortality- RR less than 1 favours 0.9% saline | |||||
| Kuppermann 2018 | RCT | 485 | RR: 0.31 (0.01, 7.45) | Moderate | Could not differentiate between IV fluids |
| Mortality- RR less than 1 favours 0.9% saline – fast rate | |||||
| Kuppermann 2018 | RCT | 238 | RR: 0.31 (0.01, 7.45) | Moderate | Could not differentiate between IV fluids |
| Mortality- RR less than 1 favours 0.9% saline – slow rate | |||||
| Kuppermann 2018 | RCT | 247 | RR not estimable due to zero event in both arms | Low | Not applicable as treatment effect could not be estimated |
| Renal failure - RR less than 1 favours 0.9% saline | |||||
| Kuppermann 2018 | RCT | 1389 | RR not estimable due to zero event in both arms | Low | Not applicable as treatment effect could not be estimated |
2 to 6 months after hospitalisation
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| IQ (in children aged 3 to 5 years) - MD greater than 0 favours 0.9% saline | |||||
| Kuppermann 2018 | RCT | 54 | MD: −2.90 (−10.22, 4.41) | Moderate | Could not differentiate between IV fluids |
| IQ (in children aged 3 to 5 years) - MD greater than 0 favours 0.9% saline – fast rate | |||||
| Kuppermann 2018 | RCT | 30 | MD: −4.00 (−13.19, 5.19) | Moderate | Could not differentiate between IV fluids |
| IQ (in children aged 3 to 5 years) - MD greater than 0 favours 0.9% saline – slow rate | |||||
| Kuppermann 2018 | RCT | 24 | MD: −1.00 (−13.09, 11.09) | Low | Could not differentiate between IV fluids |
| IQ (in children aged 6 to 18 years) - MD greater than 0 favours 0.9% saline | |||||
| Kuppermann 2018 | RCT | 768 | MD:0.48 (−1.33, 2.28) | High | Could not differentiate between IV fluids |
| IQ (in children aged 6 to 18 years) - MD greater than 0 favours 0.9% saline- fast rate | |||||
| Kuppermann 2018 | RCT | 388 | MD: 0.00 (−2.49, 2.49) | High | Could not differentiate between IV fluids |
| IQ (in children aged 6 to 18 years) - MD greater than 0 favours 0.9% saline- slow rate | |||||
| Kuppermann 2018 | RCT | 380 | 1.00 (−1.61, 6.61) | Moderate | Could not differentiate between IV fluids |
0.9% Saline vs 0.45% saline post-bolus re-hydration fluid
Outcomes during treatment of DKA
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Healthcare utilisation- Mean PICU length of stay (hours) -MD less than 0 favours 0.9% saline | |||||
| Basnet 2014 | Retrospective cohort study | 88 | MD: 2.00 (−1.01, 5.01) | Low | Could not differentiate between IV fluids |
| Rate of change of glucose (mg/dL/h) - MD greater than 0 favours 0.9% saline | |||||
| Basnet 2014 | Retrospective cohort study | 88 | MD: −7.70 (−18.02, 2.62) | Low | Could not differentiate between IV fluids |
| Change in corrected sodium from baseline (meq/L) -MD greater than 0 favours 0.9% saline | |||||
| Basnet 2014 | Retrospective cohort study | 88 | MD:3.50 (1.43, 5.57) | Low | 0.9% saline favoured |
Normal saline vs Ringer’s lactate
Outcomes during treatment of DKA
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Healthcare utilisation – mechanical ventilation – RR less than 1 favours normal saline | |||||
| Bergmann 2018 | Retrospective cohort study | 45603 | RR: 0.93 (0.59, 1.46) | Very low | Could not differentiate between IV fluids |
| Cerebral oedema – RR less than 1 favours normal saline | |||||
| Bergmann 2018 | Retrospective cohort study | 45603 | RR: 4.53 (3.68, 7.65) | Very low | Favours Ringer’s lactate |
Type 1 diabetes - All severities of DKA
75 mEq/L NaCl vs 100 mEq/L NaCl after initial rehydration
Outcomes during 1 hour of treatment
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Blood glucose levels – MD less than 0 favours 75 mEq/L of NaCl | |||||
| Savaş-Erdeve 2011 | Retrospective cohort study | 32 | MD: 0.10 (−113.06, 113.26) | Very low | Could not differentiate between IV fluids |
Outcomes during 24 hours of treatment
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Change in corrected sodium from baseline (meq/L) – MD greater than 0 favours 75 mEq/L of NaCl | |||||
| Savaş-Erdeve 2011 | Retrospective cohort study | 32 | MD: −1.00 (−3.40, 1.40) | Low | Could not differentiate between IV fluids |
| Cerebral oedema – RR less than 1 favours 75 mEq/L of NaCl | |||||
| Savaş-Erdeve 2011 | Retrospective cohort study | 32 | RR not estimable due to zero event in both arms | Very low | Not applicable as treatment effect could not be estimated |
IV+ additives
Severe DKA
IV fluid (not specified) with sodium bicarbonate vs IV fluid (not specified) with no sodium bicarbonate
Outcomes till discharge
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Duration of hospitalisation (hours) – MD less than 0 favours IV +sodium carbonate | |||||
| Green 1998 | Retrospective cohort study | 106 | MD: 16.00 (0.73, 31.27) | Very low | No sodium bicarbonate favoured |
| Cerebral oedema – RR less than 1 favours IV +sodium carbonate | |||||
| Green 1998 | Retrospective cohort study | 106 | RR: 0.86 (0.06, 13.39) | Very low | Could not differentiate between additives and no additives |
All severities of DKA
IV fluid (Lactate Ringers or Lactate Ringers with saline) with sodium bicarbonate vs IV fluid (Lactate Ringers or Lactate Ringers with saline) alone
Outcomes during treatment of DKA
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Duration of acidosis – MD less than 0 favours IV +sodium carbonate | |||||
| Mar 1981 | Retrospective cohort study | 49 | MD: −1.16 (−5.53, 3.21) | Very low | Could not differentiate between additives and no additives |
| Length of hospital stay – MD less than 0 favours IV +sodium carbonate | |||||
| Mar 1981 | Retrospective cohort study | 49 | MD: 2.05 (−2.52, 6.62) | Very low | Could not differentiate between additives and no additives |
Rate of rehydration
All severities of DKA
Fast rate (defined as replacement of half fluid deficit plus maintenance during initial 12 hours followed by the replacement of remaining deficit plus maintenance fluid during subsequent 24 hour) vs slow rate (defined as replacement of deficit plus maintenance fluids evenly during a period of 48 hours) for the replacement of deficit
Outcomes during treatment of DKA
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours fast rate | |||||
| Kuppermann 2018 | RCT | 1361 | RR: 0.77 (0.44, 1.35) | Moderate | Could not differentiate between rates |
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours fast rate – 0.45% Saline | |||||
| Kuppermann 2018 | RCT | 675 | RR: 0.91 (0.39, 2.12) | Moderate | Could not differentiate between rates |
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours 0.9% saline– slow rate | |||||
| Kuppermann 2018 | RCT | 686 | RR: 0.68 (0.32, 1.44) | Moderate | Could not differentiate between rates |
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours fast rate - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0)) | |||||
| Kuppermann 2018 | RCT | 282 | RR: 0.69 (0.34, 1.41) | Moderate | Could not differentiate between rates |
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours fast rate - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0))- 0.45% saline | |||||
| Kuppermann 2018 | RCT | 141 | RR: 0.71 (0.22, 2.31) | Moderate | Could not differentiate between rates |
| Confirmed decline in Glasgow Coma Scale score to <14 - RR less than 1 favours fast rate - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0))- 0.9% saline | |||||
| Kuppermann 2018 | RCT | 141 | RR: 0.68 (0.28, 1.66) | Moderate | Could not differentiate between rates |
| Clinically apparent brain injury - RR less than 1 favours fast rate | |||||
| Kuppermann 2018 | RCT | 1389 | RR: 0.50 (0.15, 1.65) | Low | Could not differentiate between rates |
| Clinically apparent brain injury - RR less than 1 favours fast rate – 0.45% NaCl | |||||
| Kuppermann 2018 | RCT | 689 | RR: 0.40 (0.08, 2.05) | Low | Could not differentiate between rates |
| Clinically apparent brain injury - RR less than 1 favours fast rate – 0.9% NaCl | |||||
| Kuppermann 2018 | RCT | 700 | RR: 0.66 (0.11, 3.94) | Low | Could not differentiate between rates |
| Clinically apparent brain injury - RR less than 1 favours fast rate - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0)) | |||||
| Kuppermann 2018 | RCT | 303 | RR: 0.38 (0.08, 1.87) | Low | Could not differentiate between rates |
| Clinically apparent brain injury - RR less than 1 favours fast rate - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0))- 0.45% NaCl | |||||
| Kuppermann 2018 | RCT | 152 | RR: 0.40 (0.04, 3.77) | Low | Could not differentiate between rates |
| Clinically apparent brain injury - RR less than 1 favours fast rate - in people with severe DKA (defined as with initial pH in the lowest quartile of the study group (pH <7.0))- 0.9% NaCl | |||||
| Kuppermann 2018 | RCT | 151 | RR: 0.37 (0.04, 3.44) | Low | Could not differentiate between rates |
| Mortality- RR less than 1 favours fast rate | |||||
| Kuppermann 2018 | RCT | 485 | RR: 3.10 (0.13, 75.42) | Moderate | Could not differentiate between rates |
| Mortality- RR less than 1 favours fast rate – 0.45% NaCl | |||||
| Kuppermann 2018 | RCT | 238 | RR: 3.10 (0.13, 75.42) | Moderate | Could not differentiate between rates |
| Mortality- RR less than 1 favours 0.9% saline – slow rate | |||||
| Kuppermann 2018 | RCT | 247 | RR not estimable due to zero event in both arms | Low | Not applicable as treatment effect could not be estimated |
| Renal failure - RR less than 1 favours 0.9% saline | |||||
| Kuppermann 2018 | RCT | 1389 | RR not estimable due to zero event in both arms | Low | Not applicable as treatment effect could not be estimated |
2 to 6 months after hospitalisation
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| IQ (in children aged 3 to 5 years) - MD greater than 0 favours fast rate | |||||
| Kuppermann 2018 | RCT | 54 | MD: 2.87 (−4.50, 10.23) | Moderate | Could not differentiate between rates |
| IQ (in children aged 3 to 5 years) - MD greater than 0 favours fast rate – 0.45% NaCl | |||||
| Kuppermann 2018 | RCT | 30 | 4.00 (−5.34, 13.34) | Moderate | Could not differentiate between rates |
| IQ (in children aged 3 to 5 years) - MD greater than 0 favours fast rate – 0.9% NaCl | |||||
| Kuppermann 2018 | RCT | 24 | MD: 1.00 (−10.98, 12.98) | Low | Could not differentiate between rates |
| IQ (in children aged 6 to 18 years) - MD greater than 0 favours fast rate | |||||
| Kuppermann 2018 | RCT | 768 | MD: −0.49 (−2.29, 1.32) | High | Could not differentiate between rates |
| IQ (in children aged 6 to 18 years) - MD greater than 0 fast rate- 0.4% NaCl | |||||
| Kuppermann 2018 | RCT | 388 | MD: 0.00 (−2.52, 2.52) | High | Could not differentiate between rates |
| IQ (in children aged 6 to 18 years) - MD greater than 0 favours 0.9% saline- slow rate | |||||
| Kuppermann 2018 | RCT | 380 | MD: −1.00 (−3.58, 1.58) | High | Could not differentiate between rates |
Type 1 diabetes – All severities of DKA
Rapid rate (two-thirds over first 24 hours, one-third over next 24 hours) vs slower rate (evenly over 48 hours)
Outcomes during treatment of DKA
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Treated for suspected cerebral oedema – RR less than 1 favours rapid rate | |||||
| Glaser 2013 | RCT | 18 | RR: 3.67 (0.17, 79.54) | Very low | Could not differentiate between rates |
| High risk of cerebral oedema (High risk defined as SUN in the upper quartile (≥27 mg/dL) and/ or pH in the lower quartile (≤6.97))– RR less than 1 favours rapid rate | |||||
| Glaser 2013 | RCT | 18 | RR: 2.08 (0.70, 6.19) | Very low | Could not differentiate between rates |
Fast rate (half of total required fluid over the first 12 hours of treatment and the remaining 50% over the next 24 hours) vs slow rate(total fluids delivered 2.5 times the maintenance rate and decreased to 1 to 1.5 times the maintenance rate after 24 hours
Outcomes during treatment of DKA
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Time in which acidosis resolved (hours) – MD less than 0 favours fast rate | |||||
| Felner 2001 | Retrospective cohort study | 60 | MD: 4.10 (0.79, 7.47) | Very low | Could not differentiate between rates |
| Change in sodium concentration (mmol/L)– MD greater than 0 favours fast rate | |||||
| Felner 2001 | Retrospective cohort study | 60 | MD: 0.20 (−1.93, 2.33) | Very low | Could not differentiate between rates |
| Change in chloride concentration (mmol/L)– MD greater than 0 favours fast rate | |||||
| Felner 2001 | Retrospective cohort study | 60 | MD: −0.40 (−3.72. 2.92) | Very low | Could not differentiate between rates |
Volume of rehydration
Type 1 diabetes – All severities of DKA
High volume vs low volume
Outcomes during treatment of DKA
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Metabolic normalisation – HR greater than 1 favours high volume | |||||
| Bakes 2016 | RCT | 50 | HR: 2.00 (1.01, 3.95) | Very low | High volume favoured |
| Length of treatment - HR less than 1 favours high volume | |||||
| Bakes 2016 | RCT | 50 | HR: 0.80 (0.41, 1.55) | Very low | Could not differentiate between volumes |
| Hospital discharge (hours) - HR less than 1 favours high volume | |||||
| Bakes 2016 | RCT | 50 | HR: 0.80 (0.41, 1.55) | Very low | Could not differentiate between volumes |
| Cerebral oedema – RR less than 1 favours high volume | |||||
| Bakes 2016 | RCT | 50 | RR not estimable due to zero event in both arms | Very low | Not applicable as treatment effect could not be estimated |
See appendix H for full GRADE tables
1.1.7. Economic evidence
1.1.7.1. Included studies
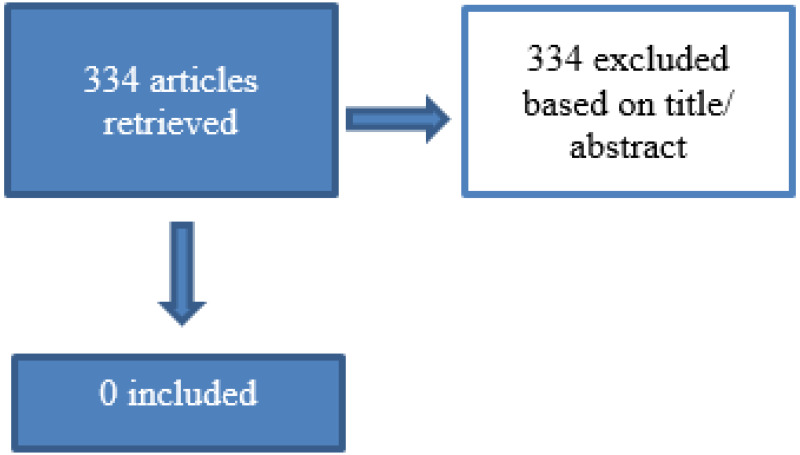
334 papers were identified for title and abstract screening, 0 were included for full text screening.
1.1.7.2. Excluded studies
See appendix F for excluded studies list.
1.1.8. Summary of included economic evidence
No economic evidence was identified for this review question.
1.1.9. Economic model
This question was not prioritised for health economic modelling.
1.1.10. Evidence statements
Evidence was also identified for which GRADE could not be applied as the evidence was presented in the form of median and interquartile range. This evidence is presented in Appendix G and summarised narratively here.
IV fluids
Moderate to severe DKA
0.9% Saline vs Hartmann’s solution as initial IV fluid – Outcomes during treatment of DKA
- Paediatric intensive care unit (PICU) or high dependency unit (HDU) stay was lower in children and young people treated with Hartmann’s solution compared to 0.9% saline solution.
0.9% Saline vs Hypertonic saline (3% NaCl) as initial IV fluid - Outcomes during treatment of DKA
- Could not differentiate average time needed for the correction of hyperglycaemia and time needed for the resolution of acidosis between children and young people who received 0.9% saline and those who received hypertonic saline.
All severities of DKA
0.9% Saline vs Plasma-Lyte-A as initial IV fluid - Outcomes during treatment of DKA
- Could not differentiate length of intensive care unit stay and length of hospital stay between children and young people who received 0.9% saline and those who received Plasma-Lyte-A.
0.9% Saline vs 0.45% saline for replacement of deficit - Outcomes during treatment of DKA
- Could not differentiate time to resolution of DKA or time to hospital discharge between children and young people who received 0.9% saline and those who received 0.45% saline.
Normal saline vs Ringers lactate - Outcomes during treatment of DKA
- Could not differentiate length of hospital stay between children and young people treated with normal saline and those who received Ringers lactate.
IV fluids
Mixed population
Fast rate vs slow rate - Outcomes during treatment of DKA
- Could not differentiate time to resolution of DKA or time to hospital discharge between children and young people who received fast rate of fluids and those who received slow rate of fluids.
1.1.11. The committee’s discussion and interpretation of the evidence
1.1.11.1. The outcomes that matter most
The committee highlighted that if DKA is not managed effectively with fluid therapy, cerebral oedema can occur which can lead to mortality. Based on this knowledge, the committee identified outcomes such as incidence of cerebral oedema and mortality as important outcomes.
1.1.11.2. The quality of the evidence
In this review, a combined search was conducted to identify studies which explored route of fluid administration for rehydration, type of fluids (including additives) that should be used for rehydration and the rate and volume these fluids should be administered. Overall, 12 studies (6 RCTs and 6 retrospective cohort studies) were included. Both RCTs and comparative observational studies started as high-quality evidence.
Overall, 7 studies (4 RCTs and 3 retrospective cohort studies) were identified which compared different IV fluids. Evidence from these studies ranged from high to very low quality. Two retrospective cohort studies were identified which compared different additives and the evidence from these studies were of very low quality. Furthermore, 3 studies were identified (2 RCTs and 1 retrospective cohort study) which compared different rates of rehydration and evidence from these studies ranged from high to very low quality. Additionally, 1 RCT was identified which compared different volumes of fluid and evidence from this study was of very low quality. These studies were downgraded through GRADE for risk of bias due to baseline differences in the 2 study arms and for not specifying DKA protocols followed. Studies were also downgraded for indirectness if the DKA protocols followed by the 2 arms of the study were different.
The review protocol specified that studies with a mixed population (children and young people with type 1 and type 2 diabetes) would be included but would be downgraded for indirectness if the data was not reported separately. Overall, 4 studies were identified (Bakes 2016, Glaser 2013, Felner 2001 and Sava-Erdeve 2011) which included participants with type 1 diabetes. The remaining studies included did not explicitly specify the patient characteristics in relation to type of diabetes and did not provide evidence split by the type of diabetes. However, the committee highlighted that DKA is rare in people with type 2 diabetes and management of DKA would not differ based on the type of diabetes. Therefore, studies which did not separate out data by type of diabetes were not downgraded. Furthermore, specific recommendations were not made based on type of diabetes.
Several studies included in the review included participants with all severities of DKA. Among these studies, the PECARN FLUID trial (Kupperman 2018) provided data on confirmed decline in the Glasgow Coma Scale (GCS) score in the whole population as well as in participants who have severe DKA (sample size = 282) which was defined as initial pH of <7.10. Compared to other studies identified in this review, the PECARN FLUID trial was the largest paediatric trial (sample size = 1389) and was considered high quality. While the study did not identify a significant difference in important outcomes in the whole population or in participants with severe DKA, the study did show that both fast and slow fluid protocols followed in the study were safe to use in children and young people with all severities of DKA. Based on this RCT, the committee drafted recommendations that covered all severities of DKA.
Subgroup analysis for different age groups (children under 5, school age children and adolescents) was planned during the review protocol stage. However, evidence was not identified for different age groups. Therefore, no specific recommendations were made based on age.
1.1.11.3. Benefits and harms
The committee noted the current recommendations on route of administration of fluids were ambiguous as in practice IV fluids are preferred in children with DKA. IV fluids can also be switched to oral fluids when the child or young person is alert and not nauseated or vomiting. Based on their clinical understanding, the committee retained the existing recommendation which states that DKA should be treated with intravenous fluids and intravenous insulin if the child or young person is not alert, is nauseated or vomiting or is clinically dehydrated. They also retained an existing recommendation that states that oral fluids should not be given to a child or young person who is receiving IV fluids for DKA unless ketosis is resolving, they are alert and they are not nauseated or vomiting.
The committee also expanded on another existing recommendation and stated that clinicians can think about stopping IV fluid therapy for DKA in a child or young person if ketosis is resolving and blood pH has reached 7.3, they are alert, and they can take oral fluids without nausea or vomiting. The committee further recommended that before stopping intravenous fluid therapy and changing to oral fluids, discussions should take place with the responsible senior paediatrician if the child or young person still has mild acidosis or ketosis. The committee also stated that this should also be dependent on the individual child’s clinical status. It should also be noted that no evidence was identified in the search which compared different routes of administration or different oral fluids for rehydration. Therefore, specific recommendations for oral fluids were not made.
When reviewing the evidence for type of fluids for rehydration, the committee noted that the evidence did not favour any of the interventions. While there were some significant results, evidence for the critical outcomes (cerebral oedema and mortality) did not favour an IV fluid for rehydration. A similar trend was also observed with evidence for rate, and volume of rehydration.
The committee noted that both fluid protocols followed in the PECARN FLUID trial were safe to use as the study did not identify a significant difference in mortality or clinically apparent brain injury. While the pathogenesis of cerebral oedema is not completely understood, the study highlighted that cerebral oedema is a feature of clinically apparent brain injury and often develops hours or days after diagnosis of brain injury. This finding suggests that cerebral oedema may be a consequence rather than a cause of brain injury. The committee further noted that the trial highlighted that restrictions to fluid administration as advised in the 2015 guideline were not necessarily required.
In line with the evidence identified from the PECARN FLUID trial and applying their clinical expertise, the committee recommended that for children and young people who are clinically dehydrated but not in shock, an initial bolus of 10ml/kg of 0.9% sodium chloride should be given over 30 minutes. This is also in line with the International Society for Paediatric and Adolescent Diabetes (ISPAD) guideline which states that resuscitation fluids should be administered over 30 to 60 minutes and if tissue perfusion is poor then initial fluid bolus should be given more rapidly, for example, over 15 to 30 minutes.
The committee further recommended that before giving more than one IV fluid bolus of 10 ml/kg 0.9% sodium chloride, it should be discussed with the responsible senior paediatrician. Additionally, a second bolus may be considered to improve tissue perfusion after reassessing their of clinical status. Separate recommendations were also developed for children and young people who present with shock.
The committee also highlighted that separate recommendations were necessary for children and young people with signs of shock. Recommendations developed in 2015 stated that IV bolus should not be given to children and young people with mild or moderate DKA and should not be routinely given to children and young people with severe DKA. The rationale provided for these recommendations further stated that fluid bolus should be avoided unless there are signs of shock associated with poor urine output or hypotension.
The committee noted that while shock is a rare occurrence in children and young people with DKA, it can occur, and such patients require more fluid boluses to improve tissue perfusion. Furthermore, the committee highlighted that restricting initial fluid boluses can result in less fluids being administered over the 48-hour period. The committee stated that this may be problematic as recent hypothesis and data suggests that brain injury may result from cerebral hypoperfusion and the effects of reperfusion and neuro-inflammation that occurs during episodes of DKA. The committee highlighted that the 2015 recommendations could have been made with the risk of cerebral oedema in mind as the previous hypothesis stated that rapid administration of IV fluids reduces serum osmolality which results in brain swelling.
Based on their clinical judgment and the RCT evidence identified in the review, particularly the PECARN FLUID trial, the committee recommended that in children and young people with DKA who have signs of shock, an initial intravenous bolus of 20 ml/kg 0.9% sodium chloride should be given as soon as possible. The committee also noted that shock may be misclassified in children and young people with moderate to severe DKA. Therefore, the committee further recommended that prolonged capillary refill, tachycardia and tachypnoea are common in children with moderate to severe DKA, but this does not mean the child or young person is in shock because these are signs of vasoconstriction caused by metabolic acidosis and hypocapnia.
The committee further highlighted that assessment of dehydration is generally poor in children and young people with DKA and the current recommendations on calculating total fluid requirement can result in less fluid being given over the 48-hour period. Based on RCT evidence identified in this review, particularly the PECARN FLUID trial, the committee retained recommendations on calculating the fluid deficit and stated that in children and young people with mild to moderate DKA, 5% dehydration should be assumed. This means that a child weighing 10kg who is 5% dehydrated would have a water deficit of 500mls. Furthermore, 10% dehydration should be assumed in children and young people with severe DKA.
The committee also highlighted that critically ill children are at a higher risk of cerebral oedema. Due to this more caution is needed when calculating fluid requirement. As the PECARN Fluid trial did not fully capture critically ill children, the committee used their expertise to recommend that the aim should be to replace the fluid deficit evenly over the first 48 hours, but in critically ill children and young people, the fluid regimen should be discussed early with the senior paediatrician or paediatric intensivist (or both), because the risk of cerebral oedema is higher. The committee further noted that it is crucial that treatment is not delayed due to the risk of cerebral oedema.
The recommendation for calculating fluid maintenance requirement was amended to include the Holliday-Segar formula. The committee noted that this formula has been shown to be safe with no adverse events and is commonly used in practice. The International Society for Paediatric and Adolescent Diabetes (ISPAD) guideline and the British Society of Paediatric Endocrinology and Diabetes (BSPED) guideline also recommend the use of this formula when calculating maintenance requirement. Additionally, the Holliday-Segar formula was also used in the PECARN FLUID trial. The committee also further stated that when calculating the total fluid requirement, any initial bolus volumes given should be subtracted from the total fluid deficit, except in children who are in shock.
The committee noted that the new recommendations will provide a more balanced approach for calculating the total fluid requirement. However, the committee did highlight that caution must be taken when calculating the fluid requirement for children and young people who are obese. Based on their clinical understanding, the committee agreed that a maximum weight of 75kg should be used in calculating fluid requirement for children and young people who are obese as this is approaching fluid requirements of adults with DKA. This will avoid excessive fluid administration and minimise risks in children and young people who are obese.
No evidence was identified for the use of potassium in the management of DKA. However, the committee highlighted that children and young people with DKA can develop hypokalaemia which occurs when there is a significant depletion of potassium in the body. Based on their clinical expertise and their understanding of the evidence on the pathophysiology of DKA the committee retained the existing recommendation but expanded it to state that 40 mmol/litre potassium chloride (or 20 mmol/500ml) should be added in all fluids (except the initial intravenous boluses) unless the child or young person with DKA has anuria or their potassium level is above the normal range. The committee also cautioned that potassium replacement should not be delayed because hypokalaemia can occur once insulin infusion begins.
The committee further highlighted that the administration of insulin and correction of acidosis, drives potassium into the cells and can lead to a fall in potassium levels. This is a major concern as this can cause cardiac arrhythmias and mortality. This means that treatment should not be delayed in children and young people with potassium levels above normal range.
Based on their clinical understanding, the committee recommended that in this population, potassium should only be added if the potassium level is less than 5.5 mmol/litre or they have passed urine, which gives the assurances that the child or young person does not have renal failure. They also recommended that for children and young people with DKA who have hypovolaemia at presentation, include potassium chloride in intravenous fluids before starting the insulin infusion.
Hypoglycaemia is another complication that can occur in children and young people with DKA. No evidence was identified in the search for the addition of glucose to IV fluids. Therefore, the committee retained the current recommendations which state that 0.9% sodium chloride should be used without added glucose for both rehydration and maintenance fluid until the plasma glucose concentration is below 14 mmol/ litre. When the glucose concentration falls below 14 mmol/litre, fluids should be changed to 0.9% sodium chloride with 5% glucose and 40mmol/litre potassium chloride.
Serum chloride concentration was included as an outcome in the review protocol. Only two studies were identified which explore this outcome. Shafi 2018 highlighted that chloride concentration was significantly lower in participants who received 0.9% saline compared to those who received hypertonic saline. Yung 2017 could not differentiate the maximum chloride concentration between participants who received 0.9% saline and those who received Hartmann’s solution.
The committee further highlighted that children and young people with DKA can develop hyperchloremic acidosis which is defined as a persisting base deficit or low bicarbonate concentration despite evidence of resolving ketosis and clinical improvement. Based on this, the committee drafted a recommendation alerting clinicians of this condition and stating that this should resolve spontaneously over time and does not require any specific management.
Additionally, serum sodium concentration was also an outcome included in the review protocol. Several studies were identified which examined sodium concentration but only one study (Basnet 2014) found that the change in corrected sodium was significantly higher with 0.9% saline compared to 0.45% saline. The committee noted that it was important to ensure that clinicians are monitoring serum sodium levels as some children and young people may be hyponatraemic, which occurs when sodium levels are low.
Based on this knowledge, the committee drafted recommendations to state that sodium levels should be monitored throughout the course of therapy and to calculate the corrected sodium initially to identify if the patient is hyponatraemic. When monitoring serum chloride levels, be aware that serum sodium should rise as DKA is treated as blood glucose falls and a falling serum sodium is a risk factor for cerebral oedema. The committee further recommended that a rapid and ongoing rise in serum sodium concentration may also suggest possible cerebral oedema, caused by the loss of free water in the urine. The committee drafted these recommendations to further support the recommendations in the ‘monitoring during therapy’ section of the guideline.
Limited evidence was identified which examined the effectiveness of adding sodium bicarbonate compared to no sodium bicarbonate. However, the evidence did not favour the use of sodium bicarbonate as an additive to IV fluids. Based on this evidence and their clinical understanding the committee agreed that sodium bicarbonate should not be routinely used. The committee also further highlighted a small number of children and young people with DKA can exhibit compromised cardiac contractility caused by life-threatening hyperkalaemia or severe acidosis. Such seriously ill children and young people can benefit from intravenous sodium bicarbonate. Based on this understanding, the committee expanded on the current recommendation to state that intravenous sodium bicarbonate should not be given to children and young people with DKA unless their cardiac contractility has been compromised by life-threatening hyperkalaemia or severe acidosis. The committee also agreed that before starting treatment, the decision should be discussed with the paediatric intensivist.
1.1.11.4. Cost effectiveness and resource use
No economic evidence was identified for this review question. The committee noted that new recommendations are in line with current practice and therefore should result in negligible cost differences. As the costs and consequences of adverse effects are severe, cost-effectiveness is driven by treatment effectiveness.
1.1.11.5. Other factors the committee took into account
The fluid protocol highlighted in the recommendations were based on RCT evidence that was identified in the search, however, the committee are aware that in some paediatric units, PlasmaLyte 148 is being used for initial resuscitation. Compared to 0.9% sodium chloride, PlasmaLyte 148 has a lower sodium and chloride content compared to 0.9% sodium chloride. It has been suggested that due to is formulation, hyperchloremic acidosis is less likely to occur.
Only one small study with only 64 partiipants (William 2020) was identified which compared PlasmaLyte (study refers to the fluid as PlasmaLyte-A) to 0.9% normal saline as initial fluid in the management of DKA in children. However, the study could not differentiate between the two fluids in outcomes such as incidence of acute kidney injury, mortality, and cerebral oedema. The committee also noted that the rates of complications identified in the study were higher than would normally be seen in NHS settings as this study was conducted in a low-middle income country.
Based on the findings of this study, the committee were unable to recommend for the use of PlasmaLyte 148 but highlighted that further research is needed to explore the effectiveness of PlasmaLyte 148 as a resuscitation fluid in the management of DKA in children and young people with diabetes. Therefore, the committee drafted a research recommendation.
The committee removed a recommendation which states that clinicians may consider inserting a urinary catheter if it is not possible to accurately measure urine output for a child or young person with DKA. While the committee agreed it was important to monitor patients, urinary catheterisation is not a commonly used in practice but may be adopted in an intensive care scenario when managing a seriously ill child or young person with DKA. As this is general guidance, the committee did not think a recommendation on urinary catheterisation was within the remit of this guideline.
The committee also highlighted that no prospective audits have been established to monitor change in practice after the 2015 DKA recommendations were produced. The committee agreed that it was important to assess the implementation of these updated recommendations in practice. As there is not an existing audit, the committee could not make any research recommendations but agreed that an audit of practice would be valuable.
Finally, the committee identified the following equality issues:
- Age – children under the age of five have a greater risk of DKA
- Race – Black and minority ethnic children present to hospital with DKA more frequently
- Sex - girls and young women are more likely to develop DKA
- Socio-economic factors - Children and young people in the most deprived areas of the UK are more likely to be hospitalised for DKA
The committee considered these equality issues but were of the opinion that these did not directly impact on fluid therapy for the management of DKA in children and young people. These equality issues would not influence the optimal route of fluid administration, type of fluid (including additives) or the rate and volume for rehydration in children and young people with DKA.
1.1.12. Recommendations supported by this evidence review
This evidence review supports recommendations 1.4.21 – 1.4.34, 1.4.39, 1.4.40 and 1.4.42 and research recommendation on effective resuscitation fluid in the management of DKA.
1.1.13. References – included studies
1.1.13.1. Effectiveness
RCTs
- Bakes, Katherine, Haukoos, Jason S, Deakyne, Sara J et al (2016) Effect of Volume of Fluid Resuscitation on Metabolic Normalization in Children Presenting in Diabetic Ketoacidosis: A Randomized Controlled Trial. The Journal of emergency medicine 50(4): 551–9 [PubMed: 26823137]
- Glaser NS, Wootton-Gorges SL, Buonocore MH et al (2013) Subclinical cerebral edema in children with diabetic ketoacidosis randomized to 2 different rehydration protocols. Pediatrics 131(1): e73 [PMC free article: PMC3529948] [PubMed: 23230065]
- Kuppermann, Nathan, Ghetti, Simona, Schunk, Jeff E et al (2018) Clinical Trial of Fluid Infusion Rates for Pediatric Diabetic Ketoacidosis. The New England journal of medicine 378(24): 2275–2287 [PMC free article: PMC6051773] [PubMed: 29897851]
- Shafi, Obeid and Kumar, Virendra (2018) Initial Fluid Therapy in Pediatric Diabetic Ketoacidosis: A comparison of Hypertonic Saline Solution and Normal Saline Solution. Pediatric endocrinology, diabetes, and metabolism 24(2): 56–64 [PubMed: 30300426]
- Williams, V., Jayashree, M., Nallasamy, K. et al (2020) 0.9% saline versus Plasma-Lyte as initial fluid in children with diabetic ketoacidosis (SPinK trial): A double-blind randomized controlled trial. Critical Care 24(1): 1 [PMC free article: PMC6939333] [PubMed: 31898531]
- Yung, Michael; Letton, Georgia; Keeley, Steve (2017) Controlled trial of Hartmann’s solution versus 0.9% saline for diabetic ketoacidosis. Journal of paediatrics and child health 53(1): 12–17 [PubMed: 28070957]
Observational studies
- Basnet, Sangita, Venepalli, Preethi K, Andoh, Jennifer et al (2014) Effect of normal saline and half normal saline on serum electrolytes during recovery phase of diabetic ketoacidosis. Journal of intensive care medicine 29(1): 38–42 [PubMed: 23753222]
- Bergmann, Kelly R, Abuzzahab, M Jennifer, Nowak, Jeffrey et al (2018) Resuscitation With Ringer’s Lactate Compared With Normal Saline for Pediatric Diabetic Ketoacidosis. Pediatric emergency care [PubMed: 30020245]
- Felner EI and White PC (2001) Improving management of diabetic ketoacidosis in children. Pediatrics 108(3): 735–740 [PubMed: 11533344]
- Green SM, Rothrock SG, Ho JD et al (1998) Failure of adjunctive bicarbonate to improve outcome in severe pediatric diabetic ketoacidosis. Annals of emergency medicine 31(1): 41–48 [PubMed: 9437340]
- Mar TJ, Traisman HS, Traisman ES et al (1981) Juvenile ketoacidosis. The use of sodium bicarbonate in the treatment of diabetic children. The Journal of the Kansas Medical Society 82(6): 282–284 [PubMed: 6788872]
- Savaş-Erdeve Ş, Berberoğlu M, Oygar P et al (2011) Efficiency of fluid treatments with different sodium concentration in children with type 1 diabetic ketoacidosis. Journal of clinical research in pediatric endocrinology 3(3): 149–153 [PMC free article: PMC3184517] [PubMed: 21911329]
1.1.13.2. Economic
None
1.1.13.3. Other
None
Appendices
Appendix A. Review protocols
Review protocol for fluid therapy for the management of DKA
Download PDF (338K)
Appendix B. Methods
Priority screening
The reviews undertaken for this guideline all made use of the priority screening functionality with the EPPI-reviewer systematic reviewing software. This uses a machine learning algorithm (specifically, an SGD classifier) to take information on features (1, 2 and 3 word blocks) in the titles and abstract of papers marked as being ‘includes’ or ‘excludes’ during the title and abstract screening process, and re-orders the remaining records from most likely to least likely to be an include, based on that algorithm. This re-ordering of the remaining records occurs every time 25 additional records have been screened. Due to the number of records identified for this review, a stopping criterion was not used when conducting screening. Therefore, all records were screened.
As an additional check to ensure this approach did not miss relevant studies, the included studies lists of included systematic reviews were searched to identify any papers not identified through the primary search. If additional studies were identified that were erroneously excluded during the priority screening process, the full database was subsequently screened.
Evidence of effectiveness of interventions
Both RCTs and cohort studies were included in this review. RCTs were considered high quality evidence. During the development of the review protocol, it was agreed that comparative observational studies would start as moderate quality evidence. However, during quality assessment ROBINS-I tool was utilised which uses one unified scale of risk of bias across study types. Therefore, observational studies were also considered high quality.
Quality assessment
Individual RCTs were quality assessed using the Cochrane Risk of Bias Tool 2.0. Cohort studies were quality assessed using the ROBINS-I tool. Each individual study was classified into one of the following groups:
- Low risk of bias – The true effect size for the study is likely to be close to the estimated effect size.
- Moderate risk of bias – There is a possibility the true effect size for the study is substantially different to the estimated effect size.
- High risk of bias – It is likely the true effect size for the study is substantially different to the estimated effect size.
- Critical risk of bias (ROBINS-I only) - It is very likely the true effect size for the study is substantially different to the estimated effect size.
Each individual study was also classified into one of three groups for directness, based on if there were concerns about the population, intervention, comparator and/or outcomes in the study and how directly these variables could address the specified review question. Studies were rated as follows:
- Direct – No important deviations from the protocol in population, intervention, comparator and/or outcomes.
- Partially indirect – Important deviations from the protocol in one of the following areas: population, intervention, comparator and/or outcomes.
- Indirect – Important deviations from the protocol in at least two of the following areas: population, intervention, comparator and/or outcomes.
Methods for combining intervention evidence
Meta-analyses of interventional data were conducted with reference to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al. 2011).
Where different studies presented continuous data measuring the same outcome but using different numerical scales (e.g. a 0-10 and a 0-100 visual analogue scale), these outcomes were all converted to the same scale before meta-analysis was conducted on the mean differences. Where outcomes measured the same underlying construct but used different instruments/metrics, data were analysed using standardised mean differences (Hedges’ g).
A pooled relative risk was calculated for dichotomous outcomes (using the Mantel–Haenszel method) reporting numbers of people having an event, and a pooled incidence rate ratio was calculated for dichotomous outcomes reporting total numbers of events. Both relative and absolute risks were presented, with absolute risks calculated by applying the relative risk to the risk in the comparator arm of the meta-analysis (calculated as the total number events in the comparator arms of studies in the meta-analysis divided by the total number of participants in the comparator arms of studies in the meta-analysis).
Fixed- and random-effects models (der Simonian and Laird) were fitted for all syntheses, with the presented analysis dependent on the degree of heterogeneity in the assembled evidence. Fixed-effects models were the preferred choice to report, but in situations where the assumption of a shared mean for fixed-effects model were clearly not met, even after appropriate pre-specified subgroup analyses were conducted, random-effects results are presented. Fixed-effects models were deemed to be inappropriate if one or both of the following conditions was met:
- Significant between study heterogeneity in methodology, population, intervention or comparator was identified by the reviewer in advance of data analysis. This decision was made and recorded before any data analysis was undertaken.
- The presence of significant statistical heterogeneity in the meta-analysis, defined as I2≥50%.
However, in cases where the results from individual pre-specified subgroup analyses are less heterogeneous (with I2 < 50%) the results from these subgroups will be reported using fixed effects models. This may lead to situations where pooled results are reported from random-effects models and subgroup results are reported from fixed-effects models.
In situations where subgroup analyses were conducted, pooled results and results for the individual subgroups are reported when there was evidence of between group heterogeneity, defined as a statistically significant test for subgroup interactions (at the 95% confidence level). Where no such evidence as identified, only pooled results are presented.
In any meta-analyses where some (but not all) of the data came from studies at critical or high risk of bias, a sensitivity analysis was conducted, excluding those studies from the analysis. Results from both the full and restricted meta-analyses are reported. Similarly, in any meta-analyses where some (but not all) of the data came from indirect studies, a sensitivity analysis was conducted, excluding those studies from the analysis.
Meta-analyses were performed in Cochrane Review Manager V5.3, with the exception of incidence rate ratio analyses which were carried out in R version 3.3.4.
Minimal clinically important differences (MIDs)
The Core Outcome Measures in Effectiveness Trials (COMET) database was searched to identify published minimal clinically important difference thresholds relevant to this guideline. Identified MIDs were assessed to ensure they had been developed and validated in a methodologically rigorous way, and were applicable to the populations, interventions and outcomes specified in this guideline.
In addition, the Guideline Committee were asked to prospectively specify any outcomes where they felt a consensus MID could be defined from their experience. In particular, any questions looking to evaluate non-inferiority (that one treatment is not meaningfully worse than another) required an MID to be defined to act as a non-inferiority margin.
No MIDs were identified through this process. Therefore, for continuous outcomes expressed as a mean difference where no other MID was available, an MID of 0.5 of the median standard deviations of the comparison group arms was used (Norman et al. 2003). For continuous outcomes expressed as a standardised mean difference where no other MID was available, an MID of 0.5 was used. For relative risks and hazard ratios, where no other MID was available, the line of no effect was used.
When decisions were made in situations where MIDs were not available, the ‘Evidence to Recommendations’ section of that review makes explicit the committee’s view of the expected clinical importance and relevance of the findings. In particular, this includes consideration of whether the whole effect of a treatment (which may be felt across multiple independent outcome domains) would be likely to be clinically meaningful, rather than simply whether each individual sub outcome might be meaningful in isolation.
GRADE for pairwise meta-analyses of interventional evidence
GRADE was used to assess the quality of evidence for the selected outcomes as specified in ‘Developing NICE guidelines: the manual (2014)’. Data from randomised controlled trials, non-randomised controlled trials and comparative observational studies were initially rated as high quality.. The quality of the evidence for each outcome was downgraded or not from this initial point, based on the criteria given in Table 1.
Table 1. Rationale for downgrading quality of evidence for intervention studies
Summary of evidence is presented in section 1.1.6. This summarises the effect size, quality of evidence and interpretation of the evidence in relation to the significance of the data.
Evidence was also identified for which GRADE could not be applied as the evidence was presented in the form of median and interquartile range. This evidence is presented in Appendix G. This evidence has been summarised narratively in section 1.1.10.
Appendix C. Literature search strategies
Clinical
| Database: MEDLINE |
|---|
| Strategy used: |
| Database: Ovid MEDLINE(R) <1946 to February 11, 2020> |
| Search Strategy: |
| ------------------------------------------------------------ |
| 1 Diabetic Ketoacidosis/ (6312) |
| 2 (DK or DKA).tw. (3081) |
| 3 (DM adj4 (keto* or acidi* or gastropare*)).tw. (71) |
| 4 or/1-3 (8271) |
| 5 exp Diabetes Mellitus/ (417002) |
| 6 diabet*.tw. (532080) |
| 7 (DM adj4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)).tw. (1609) |
| 8 lada.tw. (522) |
| 9 (dm1 or iddm or t1d* or dka).tw. (18609) |
| 10 (dm2 or t2d* or mody or niddm).tw. (30739) |
| 11 (DM adj4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II)).tw. (4115) |
| 12 (DM adj4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*)).tw. (305) |
| 13 (DM adj4 onset* adj4 (maturit* or adult* or slow*)).tw. (62) |
| 14 (DM adj4 depend* adj4 (non-insulin* or non insulin* or noninsulin*)).tw. (90) |
| 15 (DM adj4 (earl* or sudden onset or juvenile or child*)).tw. (833) |
| 16 or/5-15 (596510) |
| 17 Ketosis/ (2147) |
| 18 (keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*).tw. (120208) |
| 19 17 or 18 (120408) |
| 20 16 and 19 (12328) |
| 21 4 or 20 (15588) |
| 22 exp Fluid Therapy/ (19860) |
| 23 Rehydration Solutions/ (1444) |
| 24 Water-Electrolyte Balance/ (28875) |
| 25 Water-Electrolyte Imbalance/ (5182) |
| 26 (fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*).tw. (1017177) |
| 27 Drug Administration Routes/ (5625) |
| 28 (drug adj4 admin* adj4 route*).tw. (1229) |
| 29 (drug adj4 deliver* adj4 system*).tw. (20322) |
| 30 Administration, Oral/ (140742) |
| 31 Administration, Intravenous/ (8655) |
| 32 (oral* or intravenous or IV).tw. (1108706) |
| 33 ((vein or venous) adj4 (infus* or inject* or drip or transfus*)).tw. (9714) |
| 34 Infusions, Intravenous/ (54472) |
| 35 Infusions, Intraosseous/ (713) |
| 36 ((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) adj4 (infus* or inject* or admin* or appl*)).tw. (140740) |
| 37 infusor*.tw. (400) |
| 38 (perfusion adj4 pump*).tw. (614) |
| 39 exp Infusions, Subcutaneous/ (1166) |
| 40 hypodermoclysis.tw. (120) |
| 41 Infusion Pump/ (5300) |
| 42 Intubation, Gastrointestinal/ (9580) |
| 43 (intubat* adj4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)).tw. (551) |
| 44 (fluid bolus or two bag or ORT).tw. (1467) |
| 45 Time Factors/ (1174507) |
| 46 (time adj4 factor*).tw. (18669) |
| 47 Drug Administration Schedule/ (99082) |
| 48 (drug adj4 admin* adj4 schedul*).tw. (418) |
| 49 (drug adj4 deliver* adj4 schedul*).tw. (92) |
| 50 Sodium/ (105097) |
| 51 (sodium* or salt*).tw. (428840) |
| 52 (acetic adj4 acid).tw. (32894) |
| 53 exp Chlorides/ (133636) |
| 54 (chloride* or chlorhydrate* or hydrochloride* or monochloride*).tw. (161267) |
| 55 Glucose/ or Glucose Solution, Hypertonic/ (154527) |
| 56 (glucose or d-glucose or dextrose or l-glucose).tw. (414560) |
| 57 Saline Solution, Hypertonic/ (5551) |
| 58 Saline Solution/ or Ringer’s Lactate/ (1720) |
| 59 (saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*).tw. (227442) |
| 60 exp Bicarbonates/ (24576) |
| 61 (bicarbonate* or dicarbonate* or baros* or hydrocarbonate*).tw. (27786) |
| 62 (hydrogen adj4 carbonate*).tw. (365) |
| 63 (carbonic adj4 acid adj4 ion*).tw. (25) |
| 64 Potassium/ or Potassium Acetate/ (100804) |
| 65 (potassium or KCL or K39 or Kalium).tw. (137963) |
| 66 Phosphates/ (62400) |
| 67 (phosphate* or orthophosphate*).tw. (231304) |
| 68 or/22-67 (4489626) |
| 69 21 and 68 (7355) |
| 70 animals/ not humans/ (4639408) |
| 71 69 not 70 (6243) |
| 72 limit 71 to english language (5266) |
| 73 limit 72 to ed=20140601-20200212 (1261) |
| 74 randomized controlled trial.pt. (500124) |
| 75 randomi?ed.mp. (777830) |
| 76 placebo.mp. (191641) |
| 77 or/74-76 (828897) |
| 78 (MEDLINE or pubmed).tw. (154671) |
| 79 systematic review.tw. (112785) |
| 80 systematic review.pt. (121052) |
| 81 meta-analysis.pt. (110859) |
| 82 intervention$.ti. (119270) |
| 83 or/78-82 (362134) |
| 84 77 or 83 (1087093) |
| 85 Observational Studies as Topic/ (4691) |
| 86 Observational Study/ (74656) |
| 87 Epidemiologic Studies/ (8208) |
| 88 exp Case-Control Studies/ (1055845) |
| 89 exp Cohort Studies/ (1956602) |
| 90 Cross-Sectional Studies/ (318160) |
| 91 Controlled Before-After Studies/ (481) |
| 92 Historically Controlled Study/ (171) |
| 93 Interrupted Time Series Analysis/ (779) |
| 94 Comparative Study.pt. (1854161) |
| 95 case control$.tw. (109277) |
| 96 case series.tw. (56935) |
| 97 (cohort adj (study or studies)).tw. (160838) |
| 98 cohort analy$.tw. (6389) |
| 99 (follow up adj (study or studies)).tw. (44470) |
| 100 (observational adj (study or studies)).tw. (81969) |
| 101 longitudinal.tw. (197436) |
| 102 prospective.tw. (482132) |
| 103 retrospective.tw. (426166) |
| 104 cross sectional.tw. (272513) |
| 105 or/85-104 (4279920) |
| 106 73 and 84 (229) |
| 107 73 and 105 (407) |
| 108 106 or 107 (558) |
| Database: MEDLINE IN PROCESS |
|---|
| Strategy used: |
| Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations <1946 to February 11, 2020> |
| Search Strategy: |
| ------------------------------------------------------------ |
| 1 Diabetic Ketoacidosis/ (0) |
| 2 (DK or DKA).tw. (596) |
| 3 (DM adj4 (keto* or acidi* or gastropare*)).tw. (11) |
| 4 or/1-3 (603) |
| 5 exp Diabetes Mellitus/ (0) |
| 6 diabet*.tw. (68328) |
| 7 (DM adj4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)).tw. (290) |
| 8 lada.tw. (74) |
| 9 (dm1 or iddm or t1d* or dka).tw. (2571) |
| 10 (dm2 or t2d* or mody or niddm).tw. (6801) |
| 11 (DM adj4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II)).tw. (910) |
| 12 (DM adj4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*)).tw. (50) |
| 13 (DM adj4 onset* adj4 (maturit* or adult* or slow*)).tw. (6) |
| 14 (DM adj4 depend* adj4 (non-insulin* or non insulin* or noninsulin*)).tw. (10) |
| 15 (DM adj4 (earl* or sudden onset or juvenile or child*)).tw. (125) |
| 16 or/5-15 (68888) |
| 17 Ketosis/ (0) |
| 18 (keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*).tw. (18379) |
| 19 17 or 18 (18379) |
| 20 16 and 19 (1388) |
| 21 4 or 20 (1671) |
| 22 exp Fluid Therapy/ (0) |
| 23 Rehydration Solutions/ (0) |
| 24 Water-Electrolyte Balance/ (0) |
| 25 Water-Electrolyte Imbalance/ (0) |
| 26 (fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*).tw. (231292) |
| 27 Drug Administration Routes/ (0) |
| 28 (drug adj4 admin* adj4 route*).tw. (137) |
| 29 (drug adj4 deliver* adj4 system*).tw. (3442) |
| 30 Administration, Oral/ (0) |
| 31 Administration, Intravenous/ (0) |
| 32 (oral* or intravenous or IV).tw. (113694) |
| 33 ((vein or venous) adj4 (infus* or inject* or drip or transfus*)).tw. (739) |
| 34 Infusions, Intravenous/ (0) |
| 35 Infusions, Intraosseous/ (0) |
| 36 ((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) adj4 (infus* or inject* or admin* or appl*)).tw. (20261) |
| 37 infusor*.tw. (33) |
| 38 (perfusion adj4 pump*).tw. (24) |
| 39 exp Infusions, Subcutaneous/ (0) |
| 40 hypodermoclysis.tw. (10) |
| 41 Infusion Pump/ (0) |
| 42 Intubation, Gastrointestinal/ (0) |
| 43 (intubat* adj4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)).tw. (51) |
| 44 (fluid bolus or two bag or ORT).tw. (208) |
| 45 Time Factors/ (0) |
| 46 (time adj4 factor*).tw. (2860) |
| 47 Drug Administration Schedule/ (0) |
| 48 (drug adj4 admin* adj4 schedul*).tw. (24) |
| 49 (drug adj4 deliver* adj4 schedul*).tw. (7) |
| 50 Sodium/ (0) |
| 51 (sodium* or salt*).tw. (70278) |
| 52 (acetic adj4 acid).tw. (6320) |
| 53 exp Chlorides/ (0) |
| 54 (chloride* or chlorhydrate* or hydrochloride* or monochloride*).tw. (26669) |
| 55 Glucose/ or Glucose Solution, Hypertonic/ (0) |
| 56 (glucose or d-glucose or dextrose or l-glucose).tw. (47503) |
| 57 Saline Solution, Hypertonic/ (0) |
| 58 Saline Solution/ or Ringer’s Lactate/ (0) |
| 59 (saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*).tw. (24871) |
| 60 exp Bicarbonates/ (0) |
| 61 (bicarbonate* or dicarbonate* or baros* or hydrocarbonate*).tw. (2585) |
| 62 (hydrogen adj4 carbonate*).tw. (112) |
| 63 (carbonic adj4 acid adj4 ion*).tw. (19) |
| 64 Potassium/ or Potassium Acetate/ (0) |
| 65 (potassium or KCL or K39 or Kalium).tw. (16759) |
| 66 Phosphates/ (0) |
| 67 (phosphate* or orthophosphate*).tw. (24246) |
| 68 or/22-67 (495519) |
| 69 21 and 68 (773) |
| 70 animals/ not humans/ (0) |
| 71 69 not 70 (773) |
| 72 limit 71 to english language (759) |
| 73 limit 72 to dt=20140601-20200212 (605) |
| 74 randomized controlled trial.pt. (276) |
| 75 randomi?ed.mp. (70394) |
| 76 placebo.mp. (17332) |
| 77 or/74-76 (76533) |
| 78 (MEDLINE or pubmed).tw. (33132) |
| 79 systematic review.tw. (27200) |
| 80 systematic review.pt. (651) |
| 81 meta-analysis.pt. (43) |
| 82 intervention$.ti. (20043) |
| 83 or/78-82 (63674) |
| 84 77 or 83 (126019) |
| 85 Observational Studies as Topic/ (0) |
| 86 Observational Study/ (89) |
| 87 Epidemiologic Studies/ (0) |
| 88 exp Case-Control Studies/ (1) |
| 89 exp Cohort Studies/ (1) |
| 90 Cross-Sectional Studies/ (0) |
| 91 Controlled Before-After Studies/ (0) |
| 92 Historically Controlled Study/ (0) |
| 93 Interrupted Time Series Analysis/ (0) |
| 94 Comparative Study.pt. (45) |
| 95 case control$.tw. (13542) |
| 96 case series.tw. (11937) |
| 97 (cohort adj (study or studies)).tw. (27279) |
| 98 cohort analy$.tw. (1008) |
| 99 (follow up adj (study or studies)).tw. (3345) |
| 100 (observational adj (study or studies)).tw. (16114) |
| 101 longitudinal.tw. (32556) |
| 102 prospective.tw. (59705) |
| 103 retrospective.tw. (67413) |
| 104 cross sectional.tw. (55523) |
| 105 or/85-104 (232760) |
| 106 73 and 84 (63) |
| 107 73 and 105 (86) |
| 108 106 or 107 (139) |
| Database: MEDLINE EPUBS |
|---|
| Strategy used: |
| Database: Ovid MEDLINE(R) Epub Ahead of Print <February 11, 2020> |
| Search Strategy: |
| ------------------------------------------------------------ |
| 1 Diabetic Ketoacidosis/ (0) |
| 2 (DK or DKA).tw. (80) |
| 3 (DM adj4 (keto* or acidi* or gastropare*)).tw. (2) |
| 4 or/1-3 (81) |
| 5 exp Diabetes Mellitus/ (0) |
| 6 diabet*.tw. (9782) |
| 7 (DM adj4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)).tw. (32) |
| 8 lada.tw. (9) |
| 9 (dm1 or iddm or t1d* or dka).tw. (447) |
| 10 (dm2 or t2d* or mody or niddm).tw. (993) |
| 11 (DM adj4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II)).tw. (101) |
| 12 (DM adj4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*)).tw. (5) |
| 13 (DM adj4 onset* adj4 (maturit* or adult* or slow*)).tw. (0) |
| 14 (DM adj4 depend* adj4 (non-insulin* or non insulin* or noninsulin*)).tw. (2) |
| 15 (DM adj4 (earl* or sudden onset or juvenile or child*)).tw. (15) |
| 16 or/5-15 (9858) |
| 17 Ketosis/ (0) |
| 18 (keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*).tw. (1342) |
| 19 17 or 18 (1342) |
| 20 16 and 19 (195) |
| 21 4 or 20 (223) |
| 22 exp Fluid Therapy/ (0) |
| 23 Rehydration Solutions/ (0) |
| 24 Water-Electrolyte Balance/ (0) |
| 25 Water-Electrolyte Imbalance/ (0) |
| 26 (fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*).tw. (18082) |
| 27 Drug Administration Routes/ (0) |
| 28 (drug adj4 admin* adj4 route*).tw. (25) |
| 29 (drug adj4 deliver* adj4 system*).tw. (456) |
| 30 Administration, Oral/ (0) |
| 31 Administration, Intravenous/ (0) |
| 32 (oral* or intravenous or IV).tw. (15152) |
| 33 ((vein or venous) adj4 (infus* or inject* or drip or transfus*)).tw. (95) |
| 34 Infusions, Intravenous/ (0) |
| 35 Infusions, Intraosseous/ (0) |
| 36 ((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) adj4 (infus* or inject* or admin* or appl*)).tw. (2143) |
| 37 infusor*.tw. (1) |
| 38 (perfusion adj4 pump*).tw. (6) |
| 39 exp Infusions, Subcutaneous/ (0) |
| 40 hypodermoclysis.tw. (2) |
| 41 Infusion Pump/ (0) |
| 42 Intubation, Gastrointestinal/ (0) |
| 43 (intubat* adj4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)).tw. (7) |
| 44 (fluid bolus or two bag or ORT).tw. (36) |
| 45 Time Factors/ (0) |
| 46 (time adj4 factor*).tw. (433) |
| 47 Drug Administration Schedule/ (0) |
| 48 (drug adj4 admin* adj4 schedul*).tw. (4) |
| 49 (drug adj4 deliver* adj4 schedul*).tw. (1) |
| 50 Sodium/ (0) |
| 51 (sodium* or salt*).tw. (4951) |
| 52 (acetic adj4 acid).tw. (387) |
| 53 exp Chlorides/ (0) |
| 54 (chloride* or chlorhydrate* or hydrochloride* or monochloride*).tw. (1668) |
| 55 Glucose/ or Glucose Solution, Hypertonic/ (0) |
| 56 (glucose or d-glucose or dextrose or l-glucose).tw. (5564) |
| 57 Saline Solution, Hypertonic/ (0) |
| 58 Saline Solution/ or Ringer’s Lactate/ (0) |
| 59 (saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*).tw. (2533) |
| 60 exp Bicarbonates/ (0) |
| 61 (bicarbonate* or dicarbonate* or baros* or hydrocarbonate*).tw. (253) |
| 62 (hydrogen adj4 carbonate*).tw. (7) |
| 63 (carbonic adj4 acid adj4 ion*).tw. (0) |
| 64 Potassium/ or Potassium Acetate/ (0) |
| 65 (potassium or KCL or K39 or Kalium).tw. (1283) |
| 66 Phosphates/ (0) |
| 67 (phosphate* or orthophosphate*).tw. (2217) |
| 68 or/22-67 (46913) |
| 69 21 and 68 (132) |
| 70 animals/ not humans/ (0) |
| 71 69 not 70 (132) |
| 72 limit 71 to english language (130) |
| 73 randomized controlled trial.pt. (1) |
| 74 randomi?ed.mp. (12912) |
| 75 placebo.mp. (2942) |
| 76 or/73-75 (13891) |
| 77 (MEDLINE or pubmed).tw. (6745) |
| 78 systematic review.tw. (6529) |
| 79 systematic review.pt. (28) |
| 80 meta-analysis.pt. (26) |
| 81 intervention$.ti. (3928) |
| 82 or/77-81 (13255) |
| 83 76 or 82 (23946) |
| 84 Observational Studies as Topic/ (0) |
| 85 Observational Study/ (4) |
| 86 Epidemiologic Studies/ (0) |
| 87 exp Case-Control Studies/ (0) |
| 88 exp Cohort Studies/ (0) |
| 89 Cross-Sectional Studies/ (0) |
| 90 Controlled Before-After Studies/ (0) |
| 91 Historically Controlled Study/ (0) |
| 92 Interrupted Time Series Analysis/ (0) |
| 93 Comparative Study.pt. (0) |
| 94 case control$.tw. (2404) |
| 95 case series.tw. (1952) |
| 96 (cohort adj (study or studies)).tw. (7019) |
| 97 cohort analy$.tw. (285) |
| 98 (follow up adj (study or studies)).tw. (559) |
| 99 (observational adj (study or studies)).tw. (3337) |
| 100 longitudinal.tw. (6733) |
| 101 prospective.tw. (10883) |
| 102 retrospective.tw. (14277) |
| 103 cross sectional.tw. (8411) |
| 104 or/84-103 (43126) |
| 105 72 and 83 (12) |
| 106 72 and 104 (20) |
| 107 105 or 106 (32) |
| Database: EMBASE |
|---|
| Strategy used: |
| Database: Embase <1974 to 2020 February 11> |
| Search Strategy: |
| ------------------------------------------------------------ |
| 1 Diabetic Ketoacidosis/ (11739) |
| 2 (DK or DKA).tw. (6916) |
| 3 (DM adj4 (keto* or acidi* or gastropare*)).tw. (180) |
| 4 or/1-3 (15679) |
| 5 exp Diabetes Mellitus/ (925948) |
| 6 diabet*.tw. (903373) |
| 7 (DM adj4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)).tw. (3819) |
| 8 lada.tw. (961) |
| 9 (dm1 or iddm or t1d* or dka).tw. (37934) |
| 10 (dm2 or t2d* or mody or niddm).tw. (67126) |
| 11 (DM adj4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II)).tw. (10048) |
| 12 (DM adj4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*)).tw. (678) |
| 13 (DM adj4 onset* adj4 (maturit* or adult* or slow*)).tw. (105) |
| 14 (DM adj4 depend* adj4 (non-insulin* or non insulin* or noninsulin*)).tw. (160) |
| 15 (DM adj4 (earl* or sudden onset or juvenile or child*)).tw. (1796) |
| 16 or/5-15 (1098935) |
| 17 Ketoacidosis/ (6677) |
| 18 (keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*).tw. (175609) |
| 19 17 or 18 (177221) |
| 20 16 and 19 (21689) |
| 21 4 or 20 (28663) |
| 22 exp Fluid Therapy/ or exp Infusion fluid/ (116807) |
| 23 oral rehydration solution/ (2907) |
| 24 exp electrolyte balance/ or exp electrolyte/ (255696) |
| 25 (fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*).tw. (1492203) |
| 26 exp Drug Administration Route/ (1113204) |
| 27 (drug adj4 admin* adj4 route*).tw. (2045) |
| 28 (drug adj4 deliver* adj4 system*).tw. (34291) |
| 29 Oral Drug Administration/ (386075) |
| 30 exp Intravenous Drug Administration/ (355715) |
| 31 (oral* or intravenous or IV).tw. (1679900) |
| 32 ((vein or venous) adj4 (infus* or inject* or drip or transfus*)).tw. (16820) |
| 33 exp Intraosseous Drug Administration/ (714) |
| 34 ((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) adj4 (infus* or inject* or admin* or appl*)).tw. (211062) |
| 35 infusor*.tw. (410) |
| 36 (perfusion adj4 pump*).tw. (799) |
| 37 Subcutaneous Drug Administration/ or Hypodermoclysis/ (92822) |
| 38 hypodermoclys*.tw. (139) |
| 39 exp Infusion Pump/ (8775) |
| 40 exp Digestive Tract Intubation/ (5879) |
| 41 (intubat* adj4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)).tw. (702) |
| 42 (fluid bolus or two bag or ORT).tw. (2729) |
| 43 Time Factor/ (32342) |
| 44 (time adj4 factor*).tw. (30273) |
| 45 exp Drug Administration/ (1186551) |
| 46 (drug adj4 admin* adj4 schedul*).tw. (548) |
| 47 (drug adj4 deliver* adj4 schedul*).tw. (115) |
| 48 Acetic Acid/ or exp Inorganic Salt/ (840163) |
| 49 (sodium* or salt*).tw. (582203) |
| 50 (acetic adj4 acid).tw. (48842) |
| 51 Chloride/ (40831) |
| 52 (chloride* or chlorhydrate* or hydrochloride* or monochloride*).tw. (227860) |
| 53 Glucose/ (392979) |
| 54 (glucose or d-glucose or dextrose or l-glucose).tw. (607257) |
| 55 (saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*).tw. (325697) |
| 56 (bicarbonate* or dicarbonate* or baros* or hydrocarbonate*).tw. (38749) |
| 57 (hydrogen adj4 carbonate*).tw. (609) |
| 58 (carbonic adj4 acid adj4 ion*).tw. (29) |
| 59 (potassium or KCL or K39 or Kalium).tw. (178435) |
| 60 (phosphate* or orthophosphate*).tw. (286237) |
| 61 or/22-60 (5962035) |
| 62 21 and 61 (16102) |
| 63 nonhuman/ not human/ (4552889) |
| 64 62 not 63 (14879) |
| 65 limit 64 to english language (13389) |
| 66 limit 65 to dc=20140601-20200212 (5832) |
| 67 limit 66 to (conference abstract or conference paper or “conference review”) (2751) |
| 68 66 not 67 (3081) |
| 69 random:.tw. (1500401) |
| 70 placebo:.mp. (447102) |
| 71 double-blind:.tw. (206036) |
| 72 or/69-71 (1752190) |
| 73 (MEDLINE or pubmed).tw. (245300) |
| 74 exp systematic review/ or systematic review.tw. (281036) |
| 75 meta-analysis/ (180459) |
| 76 intervention$.ti. (192052) |
| 77 or/73-76 (626095) |
| 78 72 or 77 (2181702) |
| 79 68 and 78 (570) |
| 80 Clinical study/ (154553) |
| 81 Case control study/ (151819) |
| 82 Family study/ (25960) |
| 83 Longitudinal study/ (135655) |
| 84 Retrospective study/ (877524) |
| 85 comparative study/ (835999) |
| 86 Prospective study/ (579711) |
| 87 Randomized controlled trials/ (173699) |
| 88 86 not 87 (573647) |
| 89 Cohort analysis/ (548531) |
| 90 cohort analy$.tw. (12332) |
| 91 (Cohort adj (study or studies)).tw. (284834) |
| 92 (Case control$ adj (study or studies)).tw. (133108) |
| 93 (follow up adj (study or studies)).tw. (62104) |
| 94 (observational adj (study or studies)).tw. (159542) |
| 95 (epidemiologic$ adj (study or studies)).tw. (104201) |
| 96 (cross sectional adj (study or studies)).tw. (207345) |
| 97 case series.tw. (98892) |
| 98 prospective.tw. (832624) |
| 99 retrospective.tw. (844844) |
| 100 or/80-85, 88-99 (3885037) |
| 101 68 and 100 (681) |
| 102 79 or 101 (1117) |
| Database: COCHRANE |
|---|
| Strategy used: |
| Search Name: GU diabetes _ DKA |
| Date Run: 12/02/2020 16:00:46 |
| Comment: |
| ID Search Hits |
| #1 MeSH descriptor: [Diabetic Ketoacidosis] this term only 128 |
| #2 ((DK or DKA)):ti,ab,kw 707 |
| #3 ((DM near/4 (keto* or acidi* or gastropare*))):ti,ab,kw 12 |
| #4 #1 or #2 or #3 782 |
| #5 MeSH descriptor: [Diabetes Mellitus] explode all trees 30230 |
| #6 (diabet*):ti,ab,kw 91042 |
| #7 ((DM near/4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I))):ti,ab,kw 264 |
| #8 (lada):ti,ab,kw 66 |
| #9 ((dm1 or iddm or t1d* or dka)):ti,ab,kw 3188 |
| #10 ((dm2 or t2d* or mody or niddm)):ti,ab,kw 10004 |
| #11 ((DM near/4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II))):ti,ab,kw 1196 |
| #12 ((DM near/4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*))):ti,ab,kw 576 |
| #13 ((DM near/4 onset* near/4 (maturit* or adult* or slow*))):ti,ab,kw 0 |
| #14 ((DM near/4 depend* near/4 (non-insulin* or non insulin* or noninsulin*))):ti,ab,kw 220 |
| #15 ((DM near/4 (earl* or sudden onset or juvenile or child*))):ti,ab,kw 256 |
| #16 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 92319 |
| #17 MeSH descriptor: [Ketosis] this term only 66 |
| #18 ((keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*)):ti,ab,kw 11612 |
| #19 #17 or #18 11612 |
| #20 #16 and #19 1506 |
| #21 #4 or #20 2028 |
| #22 MeSH descriptor: [Fluid Therapy] explode all trees 1634 |
| #23 MeSH descriptor: [Rehydration Solutions] this term only 291 |
| #24 MeSH descriptor: [Water-Electrolyte Balance] this term only 714 |
| #25 MeSH descriptor: [Water-Electrolyte Imbalance] this term only 115 |
| #26 ((fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*)):ti,ab,kw 88321 |
| #27 MeSH descriptor: [Drug Administration Routes] this term only 353 |
| #28 ((drug near/4 admin* near/4 route*)):ti,ab,kw 914 |
| #29 ((drug near/4 deliver* near/4 system*)):ti,ab,kw 2811 |
| #30 MeSH descriptor: [Administration, Oral] this term only 22695 |
| #31 MeSH descriptor: [Administration, Intravenous] this term only 1048 |
| #32 ((oral* or intravenous or IV)):ti,ab,kw 284979 |
| #33 (((vein or venous) near/4 (infus* or inject* or drip or transfus*))):ti,ab,kw 1443 |
| #34 MeSH descriptor: [Infusions, Intravenous] this term only 10117 |
| #35 MeSH descriptor: [Infusions, Intraosseous] this term only 41 |
| #36 (((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) near/4 (infus* or inject* or admin* or appl*))):ti,ab,kw 28245 |
| #37 (infusor*):ti,ab,kw 72 |
| #38 ((perfusion near/4 pump*)):ti,ab,kw 66 |
| #39 MeSH descriptor: [Infusions, Subcutaneous] explode all trees 146 |
| #40 (hypodermoclysis):ti,ab,kw 32 |
| #41 MeSH descriptor: [Infusion Pumps] this term only 471 |
| #42 MeSH descriptor: [Intubation, Gastrointestinal] this term only 666 |
| #43 ((intubat* near/4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*))):ti,ab,kw 789 |
| #44 ((fluid bolus or two bag or ORT)):ti,ab,kw 2026 |
| #45 MeSH descriptor: [Time Factors] this term only 63813 |
| #46 ((time near/4 factor*)):ti,ab,kw 66337 |
| #47 MeSH descriptor: [Drug Administration Schedule] this term only 23341 |
| #48 ((drug near/4 admin* near/4 schedul*)):ti,ab,kw 23416 |
| #49 ((drug near/4 deliver* near/4 schedul*)):ti,ab,kw 18 |
| #50 MeSH descriptor: [Sodium] this term only 2062 |
| #51 ((sodium* or salt*)):ti,ab,kw 42871 |
| #52 ((acetic near/4 acid)):ti,ab,kw 1089 |
| #53 MeSH descriptor: [Chlorides] explode all trees 2940 |
| #54 ((chloride* or chlorhydrate* or hydrochloride* or monochloride*)):ti,ab,kw 27404 |
| #55 MeSH descriptor: [Glucose] this term only 3274 |
| #56 MeSH descriptor: [Glucose Solution, Hypertonic] this term only 92 |
| #57 ((glucose or d-glucose or dextrose or l-glucose)):ti,ab,kw 62627 |
| #58 MeSH descriptor: [Saline Solution, Hypertonic] this term only 499 |
| #59 MeSH descriptor: [Saline Solution] this term only 51 |
| #60 MeSH descriptor: [Ringer’s Lactate] this term only 247 |
| #61 ((saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*)):ti,ab,kw 37616 |
| #62 MeSH descriptor: [Bicarbonates] explode all trees 1252 |
| #63 ((bicarbonate* or dicarbonate* or baros* or hydrocarbonate*)):ti,ab,kw 4042 |
| #64 ((hydrogen near/4 carbonate*)):ti,ab,kw 31 |
| #65 ((carbonic near/4 acid near/4 ion*)):ti,ab,kw 2 |
| #66 MeSH descriptor: [Potassium] this term only 2179 |
| #67 MeSH descriptor: [Potassium Acetate] this term only 1 |
| #68 ((potassium or KCL or K39 or Kalium)):ti,ab,kw 11942 |
| #69 MeSH descriptor: [Phosphates] this term only 1274 |
| #70 ((phosphate* or orthophosphate*)):ti,ab,kw 12266 |
| #71 {or #22-#70} 511770 |
| #72 #23 and #71 with Cochrane Library publication date Between Jun 2014 and Feb 2020 47 |
| Database: CRD |
|---|
| Strategy used: |
| Line Search Hits |
| 1 (MeSH DESCRIPTOR Diabetic Ketoacidosis) 12 Delete |
| 2 (((DK or DKA))) 520 Delete |
| 3 ((DM) AND ((keto* or acidi* or gastropare*))) |
| 4 (#1 OR #2 OR #3) 532 Delete 3 Delete |
| 5 (MeSH DESCRIPTOR Diabetes Mellitus EXPLODE ALL TREES) 2444 Delete |
| 6 (diabet*) 4478 Delete |
| 7 ((DM) AND (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)) 29 Delete |
| 8 (lada) 1 Delete |
| 9 (dm1 or iddm or t1d* or dka) 53 Delete |
| 10 (dm2 or t2d* or mody or niddm) 83 Delete |
| 11 ((DM) AND ((“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II))) 53 Delete |
| 12 ((DM) AND ((autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*))) 8 Delete |
| 13 ((DM) AND (onset*) AND ((maturit* or adult* or slow*))) 14 Delete |
| 14 ((DM) AND (depend*) AND ((non-insulin* or non insulin* or noninsulin*))) 4 Delete |
| 15 ((DM) AND ((earl* or sudden onset or juvenile or child*))) 118 Delete |
| 16 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) 4631 Delete |
| 17 (MeSH DESCRIPTOR Ketosis) 3 Delete |
| 18 (((keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*))) 324 Delete |
| 19 (#17 OR #18) 324 Delete |
| 20 (#16 AND #19) 70 Delete |
| 21 (#4 OR #20) 587 Delete |
| 22 (MeSH DESCRIPTOR Fluid Therapy EXPLODE ALL TREES) 131 Delete |
| 23 (MeSH DESCRIPTOR Rehydration Solutions) 19 Delete |
| 24 (MeSH DESCRIPTOR Water-Electrolyte Balance) 5 Delete |
| 25 (MeSH DESCRIPTOR Water-Electrolyte Imbalance) 1 Delete |
| 26 (((fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*))) 2135 Delete |
| 27 (MeSH DESCRIPTOR Drug Administration Routes) 35 Delete |
| 28 (((drug and admin* and route*))) 311 Delete |
| 29 (((drug and deliver* and system*))) 1108 Delete |
| 30 (MeSH DESCRIPTOR Administration, Oral) 726 Delete |
| 31 (MeSH DESCRIPTOR Administration, Intravenous) 52 Delete |
| 32 (((oral* or intravenous or IV))) 7863 Delete |
| 33 (((vein or venous)) AND ((infus* or inject* or drip or transfus*))) 309 Delete |
| 34 (MeSH DESCRIPTOR Infusions, Intravenous) 351 Delete |
| 35 (MeSH DESCRIPTOR Infusions, Intraosseous) 3 Delete |
| 36 (((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip)) AND ((infus* or inject* or admin* or appl*))) 18089 Delete |
| 37 (infusor*) 4 Delete |
| 38 (perfusion and pump*) 14 Delete |
| 39 (MeSH DESCRIPTOR Infusions, Subcutaneous EXPLODE ALL TREES) 21 Delete |
| 40 (hypodermoclysis) 2 Delete |
| 41 (MeSH DESCRIPTOR Infusion Pumps) 43 Delete |
| 42 (MeSH DESCRIPTOR Intubation, Gastrointestinal) 57 Delete |
| 43 ((intubat*) AND (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)) 77 Delete |
| 44 (fluid bolus or two bag or ORT) 14 Delete |
| 45 (MeSH DESCRIPTOR Time Factors) 3076 Delete |
| 46 (time and factor*) 7112 Delete |
| 47 (MeSH DESCRIPTOR Drug Administration Schedule) 815 Delete |
| 48 (drug and admin* and schedul*) 1218 Delete |
| 49 (drug and deliver* and schedul*) 134 Delete |
| 50 (MeSH DESCRIPTOR Sodium) 13 Delete |
| 51 (sodium* or salt*) 894 Delete |
| 52 (acetic and acid) 42 Delete |
| 53 (MeSH DESCRIPTOR Chlorides EXPLODE ALL TREES) 69 Delete |
| 54 (((chloride* or chlorhydrate* or hydrochloride* or monochloride*))) 602 Delete |
| 55 (MeSH DESCRIPTOR Glucose) 56 Delete |
| 56 (MeSH DESCRIPTOR Glucose Solution, Hypertonic) 2 Delete |
| 57 (((glucose or d-glucose or dextrose or l-glucose))) 1283 Delete |
| 58 (MeSH DESCRIPTOR Saline Solution, Hypertonic) 24 Delete |
| 59 (((saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*))) 621 Delete |
| 60 (MeSH DESCRIPTOR Bicarbonates EXPLODE ALL TREES) 22 Delete |
| 61 (((bicarbonate* or dicarbonate* or baros* or hydrocarbonate*))) 73 Delete |
| 62 (((hydrogen and carbonate*))) 0 Delete |
| 63 (((carbonic and acid and ion*))) 0 Delete |
| 64 (MeSH DESCRIPTOR Potassium) 23 Delete |
| 65 (MeSH DESCRIPTOR Potassium Acetate) 0 Delete |
| 66 (((potassium or KCL or K39 or Kalium))) 205 Delete |
| 67 (MeSH DESCRIPTOR Phosphates) 32 Delete |
| 68 (((phosphate* or orthophosphate*))) 189 Delete |
| 69 (#22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68) 28822 Delete |
| 70 (#21 AND #69) 217 Delete |
| 71 ((#70) WHERE LPD FROM 01/06/2014 TO 11/02/2020) 6 Delete |
| Database: Emcare |
|---|
| Strategy used: |
| Database: Ovid Emcare <1995 to 2020 week 06> |
| Search Strategy: |
| ------------------------------------------------------------ |
| 1 Diabetic Ketoacidosis/ (3417) |
| 2 (DK or DKA).tw. (1250) |
| 3 (DM adj4 (keto* or acidi* or gastropare*)).tw. (12) |
| 4 or/1-3 (3986) |
| 5 exp Diabetes Mellitus/ (215456) |
| 6 diabet*.tw. (184022) |
| 7 (DM adj4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)).tw. (502) |
| 8 lada.tw. (177) |
| 9 (dm1 or iddm or t1d* or dka).tw. (5731) |
| 10 (dm2 or t2d* or mody or niddm).tw. (12425) |
| 11 (DM adj4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II)).tw. (1652) |
| 12 (DM adj4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*)).tw. (78) |
| 13 (DM adj4 onset* adj4 (maturit* or adult* or slow*)).tw. (10) |
| 14 (DM adj4 depend* adj4 (non-insulin* or non insulin* or noninsulin*)).tw. (29) |
| 15 (DM adj4 (earl* or sudden onset or juvenile or child*)).tw. (307) |
| 16 or/5-15 (240388) |
| 17 Ketoacidosis/ (1266) |
| 18 (keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*).tw. (16402) |
| 19 17 or 18 (16875) |
| 20 16 and 19 (3897) |
| 21 4 or 20 (5854) |
| 22 exp Fluid Therapy/ or exp Infusion fluid/ (37230) |
| 23 oral rehydration solution/ (954) |
| 24 exp electrolyte balance/ or exp electrolyte/ (46925) |
| 25 (fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*).tw. (221493) |
| 26 exp Drug Administration Route/ (86865) |
| 27 (drug adj4 admin* adj4 route*).tw. (328) |
| 28 (drug adj4 deliver* adj4 system*).tw. (3222) |
| 29 Oral Drug Administration/ (21771) |
| 30 exp Intravenous Drug Administration/ (27102) |
| 31 (oral* or intravenous or IV).tw. (290648) |
| 32 ((vein or venous) adj4 (infus* or inject* or drip or transfus*)).tw. (2146) |
| 33 exp Intraosseous Drug Administration/ (254) |
| 34 ((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) adj4 (infus* or inject* or admin* or appl*)).tw. (32164) |
| 35 infusor*.tw. (62) |
| 36 (perfusion adj4 pump*).tw. (123) |
| 37 Subcutaneous Drug Administration/ or Hypodermoclysis/ (5703) |
| 38 hypodermoclys*.tw. (69) |
| 39 exp Infusion Pump/ (2611) |
| 40 exp Digestive Tract Intubation/ (1699) |
| 41 (intubat* adj4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)).tw. (155) |
| 42 (fluid bolus or two bag or ORT).tw. (676) |
| 43 Time Factor/ (1165) |
| 44 (time adj4 factor*).tw. (7041) |
| 45 exp Drug Administration/ (97925) |
| 46 (drug adj4 admin* adj4 schedul*).tw. (94) |
| 47 (drug adj4 deliver* adj4 schedul*).tw. (11) |
| 48 Acetic Acid/ or exp Inorganic Salt/ (124456) |
| 49 (sodium* or salt*).tw. (52046) |
| 50 (acetic adj4 acid).tw. (3522) |
| 51 Chloride/ (3547) |
| 52 (chloride* or chlorhydrate* or hydrochloride* or monochloride*).tw. (16131) |
| 53 Glucose/ (81096) |
| 54 (glucose or d-glucose or dextrose or l-glucose).tw. (88868) |
| 55 (saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*).tw. (43420) |
| 56 (bicarbonate* or dicarbonate* or baros* or hydrocarbonate*).tw. (3899) |
| 57 (hydrogen adj4 carbonate*).tw. (39) |
| 58 (carbonic adj4 acid adj4 ion*).tw. (1) |
| 59 (potassium or KCL or K39 or Kalium).tw. (14715) |
| 60 (phosphate* or orthophosphate*).tw. (24140) |
| 61 or/22-60 (828600) |
| 62 21 and 61 (3250) |
| 63 nonhuman/ not human/ (392209) |
| 64 62 not 63 (3135) |
| 65 limit 64 to english language (2973) |
| 66 limit 65 to dc=20140601-20200212 (1301) |
| 67 limit 66 to (conference abstract or conference paper or “conference review”) (31) |
| 68 66 not 67 (1270) |
| 69 random:.tw. (422338) |
| 70 placebo:.mp. (110102) |
| 71 double-blind:.tw. (47817) |
| 72 or/69-71 (478544) |
| 73 (MEDLINE or pubmed).tw. (88546) |
| 74 exp systematic review/ or systematic review.tw. (110922) |
| 75 meta-analysis/ (55911) |
| 76 intervention$.ti. (81102) |
| 77 or/73-76 (235827) |
| 78 72 or 77 (642558) |
| 79 68 and 78 (265) |
| 80 Clinical study/ (45399) |
| 81 Case control study/ (33617) |
| 82 Family study/ (8749) |
| 83 Longitudinal study/ (53637) |
| 84 Retrospective study/ (198020) |
| 85 comparative study/ (116407) |
| 86 Prospective study/ (160163) |
| 87 Randomized controlled trials/ (63538) |
| 88 86 not 87 (158171) |
| 89 Cohort analysis/ (163284) |
| 90 cohort analy$.tw. (3267) |
| 91 (Cohort adj (study or studies)).tw. (91295) |
| 92 (Case control$ adj (study or studies)).tw. (32205) |
| 93 (follow up adj (study or studies)).tw. (14930) |
| 94 (observational adj (study or studies)).tw. (47431) |
| 95 (epidemiologic$ adj (study or studies)).tw. (24505) |
| 96 (cross sectional adj (study or studies)).tw. (75459) |
| 97 case series.tw. (25526) |
| 98 prospective.tw. (213336) |
| 99 retrospective.tw. (181786) |
| 100 or/80-85, 88-99 (895064) |
| 101 68 and 100 (270) |
| 102 79 or 101 (478) |
| MHRA |
|---|
| Search terms: |
| Saline |
| “Hartmann’s” |
| “Ringer’s Lactate” |
| “Sodium Chloride” |
| Salt |
| “Glucose solution” |
| Dextrose |
| NaCL or Na-cl |
| Bicarbonate |
| Dicarbonate |
| Baros |
| Hydrocarbonate |
| “Hydrogen carbonate” |
| “carbonic acid” |
| Potassium |
| KCL |
| K39 |
| Kalium |
| Phosphate |
| Orthophosphate |
Health Economics
| Database: MEDLINE |
|---|
| Strategy used: |
| Database: Ovid MEDLINE(R) <1946 to February 12, 2020> |
| Search Strategy: |
| ------------------------------------------------------------ |
| 1 Diabetic Ketoacidosis/ (6312) |
| 2 (DK or DKA).tw. (3082) |
| 3 (DM adj4 (keto* or acidi* or gastropare*)).tw. (71) |
| 4 or/1-3 (8272) |
| 5 exp Diabetes Mellitus/ (417196) |
| 6 diabet*.tw. (532357) |
| 7 (DM adj4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)).tw. (1610) |
| 8 lada.tw. (523) |
| 9 (dm1 or iddm or t1d* or dka).tw. (18614) |
| 10 (dm2 or t2d* or mody or niddm).tw. (30765) |
| 11 (DM adj4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II)).tw. (4120) |
| 12 (DM adj4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*)).tw. (305) |
| 13 (DM adj4 onset* adj4 (maturit* or adult* or slow*)).tw. (62) |
| 14 (DM adj4 depend* adj4 (non-insulin* or non insulin* or noninsulin*)).tw. (90) |
| 15 (DM adj4 (earl* or sudden onset or juvenile or child*)).tw. (833) |
| 16 or/5-15 (596801) |
| 17 Ketosis/ (2148) |
| 18 (keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*).tw. (120232) |
| 19 17 or 18 (120432) |
| 20 16 and 19 (12332) |
| 21 4 or 20 (15593) |
| 22 exp Fluid Therapy/ (19871) |
| 23 Rehydration Solutions/ (1444) |
| 24 Water-Electrolyte Balance/ (28879) |
| 25 Water-Electrolyte Imbalance/ (5183) |
| 26 (fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*).tw. (1017456) |
| 27 Drug Administration Routes/ (5627) |
| 28 (drug adj4 admin* adj4 route*).tw. (1229) |
| 29 (drug adj4 deliver* adj4 system*).tw. (20333) |
| 30 Administration, Oral/ (140774) |
| 31 Administration, Intravenous/ (8664) |
| 32 (oral* or intravenous or IV).tw. (1109028) |
| 33 ((vein or venous) adj4 (infus* or inject* or drip or transfus*)).tw. (9716) |
| 34 Infusions, Intravenous/ (54480) |
| 35 Infusions, Intraosseous/ (713) |
| 36 ((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) adj4 (infus* or inject* or admin* or appl*)).tw. (140778) |
| 37 infusor*.tw. (400) |
| 38 (perfusion adj4 pump*).tw. (614) |
| 39 exp Infusions, Subcutaneous/ (1166) |
| 40 hypodermoclysis.tw. (120) |
| 41 Infusion Pump/ (5300) |
| 42 Intubation, Gastrointestinal/ (9581) |
| 43 (intubat* adj4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)).tw. (551) |
| 44 (fluid bolus or two bag or ORT).tw. (1467) |
| 45 Time Factors/ (1174766) |
| 46 (time adj4 factor*).tw. (18673) |
| 47 Drug Administration Schedule/ (99108) |
| 48 (drug adj4 admin* adj4 schedul*).tw. (418) |
| 49 (drug adj4 deliver* adj4 schedul*).tw. (92) |
| 50 Sodium/ (105105) |
| 51 (sodium* or salt*).tw. (428926) |
| 52 (acetic adj4 acid).tw. (32901) |
| 53 exp Chlorides/ (133646) |
| 54 (chloride* or chlorhydrate* or hydrochloride* or monochloride*).tw. (161298) |
| 55 Glucose/ or Glucose Solution, Hypertonic/ (154551) |
| 56 (glucose or d-glucose or dextrose or l-glucose).tw. (414689) |
| 57 Saline Solution, Hypertonic/ (5552) |
| 58 Saline Solution/ or Ringer’s Lactate/ (1722) |
| 59 (saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*).tw. (227485) |
| 60 exp Bicarbonates/ (24577) |
| 61 (bicarbonate* or dicarbonate* or baros* or hydrocarbonate*).tw. (27789) |
| 62 (hydrogen adj4 carbonate*).tw. (365) |
| 63 (carbonic adj4 acid adj4 ion*).tw. (25) |
| 64 Potassium/ or Potassium Acetate/ (100807) |
| 65 (potassium or KCL or K39 or Kalium).tw. (137981) |
| 66 Phosphates/ (62408) |
| 67 (phosphate* or orthophosphate*).tw. (231352) |
| 68 or/22-67 (4490732) |
| 69 21 and 68 (7357) |
| 70 animals/ not humans/ (4640070) |
| 71 69 not 70 (6245) |
| 72 limit 71 to english language (5268) |
| 73 limit 72 to ed=20140601-20200213 (1263) |
| 74 Economics/ (27130) |
| 75 exp “Costs and Cost Analysis”/ (232579) |
| 76 Economics, Dental/ (1910) |
| 77 exp Economics, Hospital/ (24220) |
| 78 exp Economics, Medical/ (14162) |
| 79 Economics, Nursing/ (3996) |
| 80 Economics, Pharmaceutical/ (2913) |
| 81 Budgets/ (11224) |
| 82 exp Models, Economic/ (14715) |
| 83 Markov Chains/ (13986) |
| 84 Monte Carlo Method/ (27788) |
| 85 Decision Trees/ (10899) |
| 86 econom$.tw. (230976) |
| 87 cba.tw. (9705) |
| 88 cea.tw. (20202) |
| 89 cua.tw. (975) |
| 90 markov$.tw. (17479) |
| 91 (monte adj carlo).tw. (29286) |
| 92 (decision adj3 (tree$ or analys$)).tw. (12893) |
| 93 (cost or costs or costing$ or costly or costed).tw. (447358) |
| 94 (price$ or pricing$).tw. (32599) |
| 95 budget$.tw. (23162) |
| 96 expenditure$.tw. (48111) |
| 97 (value adj3 (money or monetary)).tw. (2036) |
| 98 (pharmacoeconomic$ or (pharmaco adj economic$)).tw. (3440) |
| 99 or/74-98 (902730) |
| 100 “Quality of Life”/ (188197) |
| 101 quality of life.tw. (221871) |
| 102 “Value of Life”/ (5683) |
| 103 Quality-Adjusted Life Years/ (11827) |
| 104 quality adjusted life.tw. (10401) |
| 105 (qaly$ or qald$ or qale$ or qtime$).tw. (8539) |
| 106 disability adjusted life.tw. (2568) |
| 107 daly$.tw. (2345) |
| 108 Health Status Indicators/ (23206) |
| 109 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. (21931) |
| 110 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. (1293) |
| 111 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. (4719) |
| 112 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. (28) |
| 113 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. (377) |
| 114 (euroqol or euro qol or eq5d or eq 5d).tw. (8494) |
| 115 (qol or hql or hqol or hrqol).tw. (42347) |
| 116 (hye or hyes).tw. (60) |
| 117 health$ year$ equivalent$.tw. (38) |
| 118 utilit$.tw. (166224) |
| 119 (hui or hui1 or hui2 or hui3).tw. (1259) |
| 120 disutili$.tw. (371) |
| 121 rosser.tw. (92) |
| 122 quality of wellbeing.tw. (13) |
| 123 quality of well-being.tw. (378) |
| 124 qwb.tw. (189) |
| 125 willingness to pay.tw. (4256) |
| 126 standard gamble$.tw. (774) |
| 127 time trade off.tw. (1013) |
| 128 time tradeoff.tw. (228) |
| 129 tto.tw. (876) |
| 130 or/100-129 (477925) |
| 131 99 or 130 (1314376) |
| 132 73 and 131 (87) |
| Database: MEDLINE IN PROCESS |
|---|
| Strategy used: |
| Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations <1946 to February 12, 2020> |
| Search Strategy: |
| ------------------------------------------------------------ |
| 1 Diabetic Ketoacidosis/ (0) |
| 2 (DK or DKA).tw. (596) |
| 3 (DM adj4 (keto* or acidi* or gastropare*)).tw. (11) |
| 4 or/1-3 (603) |
| 5 exp Diabetes Mellitus/ (0) |
| 6 diabet*.tw. (68468) |
| 7 (DM adj4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)).tw. (290) |
| 8 lada.tw. (74) |
| 9 (dm1 or iddm or t1d* or dka).tw. (2575) |
| 10 (dm2 or t2d* or mody or niddm).tw. (6816) |
| 11 (DM adj4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II)).tw. (910) |
| 12 (DM adj4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*)).tw. (50) |
| 13 (DM adj4 onset* adj4 (maturit* or adult* or slow*)).tw. (6) |
| 14 (DM adj4 depend* adj4 (non-insulin* or non insulin* or noninsulin*)).tw. (10) |
| 15 (DM adj4 (earl* or sudden onset or juvenile or child*)).tw. (125) |
| 16 or/5-15 (69028) |
| 17 Ketosis/ (0) |
| 18 (keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*).tw. (18401) |
| 19 17 or 18 (18401) |
| 20 16 and 19 (1389) |
| 21 4 or 20 (1672) |
| 22 exp Fluid Therapy/ (0) |
| 23 Rehydration Solutions/ (0) |
| 24 Water-Electrolyte Balance/ (0) |
| 25 Water-Electrolyte Imbalance/ (0) |
| 26 (fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*).tw. (231568) |
| 27 Drug Administration Routes/ (0) |
| 28 (drug adj4 admin* adj4 route*).tw. (137) |
| 29 (drug adj4 deliver* adj4 system*).tw. (3456) |
| 30 Administration, Oral/ (0) |
| 31 Administration, Intravenous/ (0) |
| 32 (oral* or intravenous or IV).tw. (113920) |
| 33 ((vein or venous) adj4 (infus* or inject* or drip or transfus*)).tw. (743) |
| 34 Infusions, Intravenous/ (0) |
| 35 Infusions, Intraosseous/ (0) |
| 36 ((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) adj4 (infus* or inject* or admin* or appl*)).tw. (20301) |
| 37 infusor*.tw. (33) |
| 38 (perfusion adj4 pump*).tw. (24) |
| 39 exp Infusions, Subcutaneous/ (0) |
| 40 hypodermoclysis.tw. (10) |
| 41 Infusion Pump/ (0) |
| 42 Intubation, Gastrointestinal/ (0) |
| 43 (intubat* adj4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)).tw. (51) |
| 44 (fluid bolus or two bag or ORT).tw. (208) |
| 45 Time Factors/ (0) |
| 46 (time adj4 factor*).tw. (2863) |
| 47 Drug Administration Schedule/ (0) |
| 48 (drug adj4 admin* adj4 schedul*).tw. (24) |
| 49 (drug adj4 deliver* adj4 schedul*).tw. (7) |
| 50 Sodium/ (0) |
| 51 (sodium* or salt*).tw. (70384) |
| 52 (acetic adj4 acid).tw. (6327) |
| 53 exp Chlorides/ (0) |
| 54 (chloride* or chlorhydrate* or hydrochloride* or monochloride*).tw. (26712) |
| 55 Glucose/ or Glucose Solution, Hypertonic/ (0) |
| 56 (glucose or d-glucose or dextrose or l-glucose).tw. (47584) |
| 57 Saline Solution, Hypertonic/ (0) |
| 58 Saline Solution/ or Ringer’s Lactate/ (0) |
| 59 (saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*).tw. (24916) |
| 60 exp Bicarbonates/ (0) |
| 61 (bicarbonate* or dicarbonate* or baros* or hydrocarbonate*).tw. (2590) |
| 62 (hydrogen adj4 carbonate*).tw. (112) |
| 63 (carbonic adj4 acid adj4 ion*).tw. (19) |
| 64 Potassium/ or Potassium Acetate/ (0) |
| 65 (potassium or KCL or K39 or Kalium).tw. (16781) |
| 66 Phosphates/ (0) |
| 67 (phosphate* or orthophosphate*).tw. (24303) |
| 68 or/22-67 (496279) |
| 69 21 and 68 (774) |
| 70 animals/ not humans/ (0) |
| 71 69 not 70 (774) |
| 72 limit 71 to english language (760) |
| 73 limit 72 to dt=20140601-20200213 (606) |
| 74 Economics/ (0) |
| 75 exp “Costs and Cost Analysis”/ (0) |
| 76 Economics, Dental/ (0) |
| 77 exp Economics, Hospital/ (0) |
| 78 exp Economics, Medical/ (0) |
| 79 Economics, Nursing/ (0) |
| 80 Economics, Pharmaceutical/ (0) |
| 81 Budgets/ (0) |
| 82 exp Models, Economic/ (0) |
| 83 Markov Chains/ (0) |
| 84 Monte Carlo Method/ (0) |
| 85 Decision Trees/ (0) |
| 86 econom$.tw. (43397) |
| 87 cba.tw. (411) |
| 88 cea.tw. (1838) |
| 89 cua.tw. (197) |
| 90 markov$.tw. (5488) |
| 91 (monte adj carlo).tw. (16562) |
| 92 (decision adj3 (tree$ or analys$)).tw. (2309) |
| 93 (cost or costs or costing$ or costly or costed).tw. (92917) |
| 94 (price$ or pricing$).tw. (5667) |
| 95 budget$.tw. (4861) |
| 96 expenditure$.tw. (6223) |
| 97 (value adj3 (money or monetary)).tw. (346) |
| 98 (pharmacoeconomic$ or (pharmaco adj economic$)).tw. (517) |
| 99 or/74-98 (160962) |
| 100 “Quality of Life”/ (0) |
| 101 quality of life.tw. (37218) |
| 102 “Value of Life”/ (0) |
| 103 Quality-Adjusted Life Years/ (0) |
| 104 quality adjusted life.tw. (1621) |
| 105 (qaly$ or qald$ or qale$ or qtime$).tw. (1387) |
| 106 disability adjusted life.tw. (489) |
| 107 daly$.tw. (451) |
| 108 Health Status Indicators/ (0) |
| 109 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. (2583) |
| 110 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. (746) |
| 111 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. (712) |
| 112 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. (5) |
| 113 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. (19) |
| 114 (euroqol or euro qol or eq5d or eq 5d).tw. (1605) |
| 115 (qol or hql or hqol or hrqol).tw. (7139) |
| 116 (hye or hyes).tw. (8) |
| 117 health$ year$ equivalent$.tw. (2) |
| 118 utilit$.tw. (30118) |
| 119 (hui or hui1 or hui2 or hui3).tw. (175) |
| 120 disutili$.tw. (71) |
| 121 rosser.tw. (5) |
| 122 quality of wellbeing.tw. (7) |
| 123 quality of well-being.tw. (26) |
| 124 qwb.tw. (11) |
| 125 willingness to pay.tw. (925) |
| 126 standard gamble$.tw. (61) |
| 127 time trade off.tw. (118) |
| 128 time tradeoff.tw. (17) |
| 129 tto.tw. (121) |
| 130 or/100-129 (69607) |
| 131 99 or 130 (221378) |
| 132 73 and 131 (43) |
| Database: MEDLINE EPUBS |
|---|
| Strategy used: |
| Database: Ovid MEDLINE(R) Epub Ahead of Print <February 12, 2020> |
| Search Strategy: |
| ------------------------------------------------------------ |
| 1 Diabetic Ketoacidosis/ (0) |
| 2 (DK or DKA).tw. (80) |
| 3 (DM adj4 (keto* or acidi* or gastropare*)).tw. (2) |
| 4 or/1-3 (81) |
| 5 exp Diabetes Mellitus/ (0) |
| 6 diabet*.tw. (9756) |
| 7 (DM adj4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)).tw. (33) |
| 8 lada.tw. (10) |
| 9 (dm1 or iddm or t1d* or dka).tw. (445) |
| 10 (dm2 or t2d* or mody or niddm).tw. (992) |
| 11 (DM adj4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II)).tw. (102) |
| 12 (DM adj4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*)).tw. (5) |
| 13 (DM adj4 onset* adj4 (maturit* or adult* or slow*)).tw. (0) |
| 14 (DM adj4 depend* adj4 (non-insulin* or non insulin* or noninsulin*)).tw. (2) |
| 15 (DM adj4 (earl* or sudden onset or juvenile or child*)).tw. (15) |
| 16 or/5-15 (9833) |
| 17 Ketosis/ (0) |
| 18 (keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*).tw. (1341) |
| 19 17 or 18 (1341) |
| 20 16 and 19 (194) |
| 21 4 or 20 (222) |
| 22 exp Fluid Therapy/ (0) |
| 23 Rehydration Solutions/ (0) |
| 24 Water-Electrolyte Balance/ (0) |
| 25 Water-Electrolyte Imbalance/ (0) |
| 26 (fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*).tw. (18023) |
| 27 Drug Administration Routes/ (0) |
| 28 (drug adj4 admin* adj4 route*).tw. (26) |
| 29 (drug adj4 deliver* adj4 system*).tw. (455) |
| 30 Administration, Oral/ (0) |
| 31 Administration, Intravenous/ (0) |
| 32 (oral* or intravenous or IV).tw. (15145) |
| 33 ((vein or venous) adj4 (infus* or inject* or drip or transfus*)).tw. (94) |
| 34 Infusions, Intravenous/ (0) |
| 35 Infusions, Intraosseous/ (0) |
| 36 ((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) adj4 (infus* or inject* or admin* or appl*)).tw. (2130) |
| 37 infusor*.tw. (1) |
| 38 (perfusion adj4 pump*).tw. (6) |
| 39 exp Infusions, Subcutaneous/ (0) |
| 40 hypodermoclysis.tw. (2) |
| 41 Infusion Pump/ (0) |
| 42 Intubation, Gastrointestinal/ (0) |
| 43 (intubat* adj4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)).tw. (7) |
| 44 (fluid bolus or two bag or ORT).tw. (37) |
| 45 Time Factors/ (0) |
| 46 (time adj4 factor*).tw. (433) |
| 47 Drug Administration Schedule/ (0) |
| 48 (drug adj4 admin* adj4 schedul*).tw. (4) |
| 49 (drug adj4 deliver* adj4 schedul*).tw. (1) |
| 50 Sodium/ (0) |
| 51 (sodium* or salt*).tw. (4936) |
| 52 (acetic adj4 acid).tw. (385) |
| 53 exp Chlorides/ (0) |
| 54 (chloride* or chlorhydrate* or hydrochloride* or monochloride*).tw. (1656) |
| 55 Glucose/ or Glucose Solution, Hypertonic/ (0) |
| 56 (glucose or d-glucose or dextrose or l-glucose).tw. (5562) |
| 57 Saline Solution, Hypertonic/ (0) |
| 58 Saline Solution/ or Ringer’s Lactate/ (0) |
| 59 (saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*).tw. (2523) |
| 60 exp Bicarbonates/ (0) |
| 61 (bicarbonate* or dicarbonate* or baros* or hydrocarbonate*).tw. (251) |
| 62 (hydrogen adj4 carbonate*).tw. (7) |
| 63 (carbonic adj4 acid adj4 ion*).tw. (0) |
| 64 Potassium/ or Potassium Acetate/ (0) |
| 65 (potassium or KCL or K39 or Kalium).tw. (1282) |
| 66 Phosphates/ (0) |
| 67 (phosphate* or orthophosphate*).tw. (2199) |
| 68 or/22-67 (46812) |
| 69 21 and 68 (131) |
| 70 animals/ not humans/ (0) |
| 71 69 not 70 (131) |
| 72 limit 71 to english language (129) |
| 73 Economics/ (0) |
| 74 exp “Costs and Cost Analysis”/ (0) |
| 75 Economics, Dental/ (0) |
| 76 exp Economics, Hospital/ (0) |
| 77 exp Economics, Medical/ (0) |
| 78 Economics, Nursing/ (0) |
| 79 Economics, Pharmaceutical/ (0) |
| 80 Budgets/ (0) |
| 81 exp Models, Economic/ (0) |
| 82 Markov Chains/ (0) |
| 83 Monte Carlo Method/ (0) |
| 84 Decision Trees/ (0) |
| 85 econom$.tw. (5922) |
| 86 cba.tw. (64) |
| 87 cea.tw. (330) |
| 88 cua.tw. (16) |
| 89 markov$.tw. (725) |
| 90 (monte adj carlo).tw. (1181) |
| 91 (decision adj3 (tree$ or analys$)).tw. (412) |
| 92 (cost or costs or costing$ or costly or costed).tw. (12195) |
| 93 (price$ or pricing$).tw. (871) |
| 94 budget$.tw. (521) |
| 95 expenditure$.tw. (1117) |
| 96 (value adj3 (money or monetary)).tw. (71) |
| 97 (pharmacoeconomic$ or (pharmaco adj economic$)).tw. (47) |
| 98 or/73-97 (20059) |
| 99 “Quality of Life”/ (0) |
| 100 quality of life.tw. (6798) |
| 101 “Value of Life”/ (0) |
| 102 Quality-Adjusted Life Years/ (0) |
| 103 quality adjusted life.tw. (401) |
| 104 (qaly$ or qald$ or qale$ or qtime$).tw. (348) |
| 105 disability adjusted life.tw. (108) |
| 106 daly$.tw. (92) |
| 107 Health Status Indicators/ (0) |
| 108 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. (454) |
| 109 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. (42) |
| 110 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. (164) |
| 111 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. (0) |
| 112 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. (4) |
| 113 (euroqol or euro qol or eq5d or eq 5d).tw. (343) |
| 114 (qol or hql or hqol or hrqol).tw. (1323) |
| 115 (hye or hyes).tw. (1) |
| 116 health$ year$ equivalent$.tw. (0) |
| 117 utilit$.tw. (4612) |
| 118 (hui or hui1 or hui2 or hui3).tw. (24) |
| 119 disutili$.tw. (12) |
| 120 rosser.tw. (0) |
| 121 quality of wellbeing.tw. (1) |
| 122 quality of well-being.tw. (6) |
| 123 qwb.tw. (4) |
| 124 willingness to pay.tw. (161) |
| 125 standard gamble$.tw. (8) |
| 126 time trade off.tw. (18) |
| 127 time tradeoff.tw. (3) |
| 128 tto.tw. (19) |
| 129 or/99-128 (11694) |
| 130 98 or 129 (29991) |
| 131 72 and 130 (8) |
| Database: EMBASE |
|---|
| Strategy used: |
| Database: Embase <1974 to 2020 February 12> |
| Search Strategy: |
| ------------------------------------------------------------ |
| 1 Diabetic Ketoacidosis/ (11739) |
| 2 (DK or DKA).tw. (6916) |
| 3 (DM adj4 (keto* or acidi* or gastropare*)).tw. (180) |
| 4 or/1-3 (15679) |
| 5 exp Diabetes Mellitus/ (925948) |
| 6 diabet*.tw. (903373) |
| 7 (DM adj4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)).tw. (3819) |
| 8 lada.tw. (961) |
| 9 (dm1 or iddm or t1d* or dka).tw. (37934) |
| 10 (dm2 or t2d* or mody or niddm).tw. (67126) |
| 11 (DM adj4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II)).tw. (10048) |
| 12 (DM adj4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*)).tw. (678) |
| 13 (DM adj4 onset* adj4 (maturit* or adult* or slow*)).tw. (105) |
| 14 (DM adj4 depend* adj4 (non-insulin* or non insulin* or noninsulin*)).tw. (160) |
| 15 (DM adj4 (earl* or sudden onset or juvenile or child*)).tw. (1796) |
| 16 or/5-15 (1098935) |
| 17 Ketoacidosis/ (6677) |
| 18 (keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*).tw. (175609) |
| 19 17 or 18 (177221) |
| 20 16 and 19 (21689) |
| 21 4 or 20 (28663) |
| 22 exp Fluid Therapy/ or exp Infusion fluid/ (116807) |
| 23 oral rehydration solution/ (2907) |
| 24 exp electrolyte balance/ or exp electrolyte/ (255696) |
| 25 (fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*).tw. (1492203) |
| 26 exp Drug Administration Route/ (1113204) |
| 27 (drug adj4 admin* adj4 route*).tw. (2045) |
| 28 (drug adj4 deliver* adj4 system*).tw. (34291) |
| 29 Oral Drug Administration/ (386075) |
| 30 exp Intravenous Drug Administration/ (355715) |
| 31 (oral* or intravenous or IV).tw. (1679900) |
| 32 ((vein or venous) adj4 (infus* or inject* or drip or transfus*)).tw. (16820) |
| 33 exp Intraosseous Drug Administration/ (714) |
| 34 ((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) adj4 (infus* or inject* or admin* or appl*)).tw. (211062) |
| 35 infusor*.tw. (410) |
| 36 (perfusion adj4 pump*).tw. (799) |
| 37 Subcutaneous Drug Administration/ or Hypodermoclysis/ (92822) |
| 38 hypodermoclys*.tw. (139) |
| 39 exp Infusion Pump/ (8775) |
| 40 exp Digestive Tract Intubation/ (5879) |
| 41 (intubat* adj4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)).tw. (702) |
| 42 (fluid bolus or two bag or ORT).tw. (2729) |
| 43 Time Factor/ (32342) |
| 44 (time adj4 factor*).tw. (30273) |
| 45 exp Drug Administration/ (1186551) |
| 46 (drug adj4 admin* adj4 schedul*).tw. (548) |
| 47 (drug adj4 deliver* adj4 schedul*).tw. (115) |
| 48 Acetic Acid/ or exp Inorganic Salt/ (840163) |
| 49 (sodium* or salt*).tw. (582203) |
| 50 (acetic adj4 acid).tw. (48842) |
| 51 Chloride/ (40831) |
| 52 (chloride* or chlorhydrate* or hydrochloride* or monochloride*).tw. (227860) |
| 53 Glucose/ (392979) |
| 54 (glucose or d-glucose or dextrose or l-glucose).tw. (607257) |
| 55 (saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*).tw. (325697) |
| 56 (bicarbonate* or dicarbonate* or baros* or hydrocarbonate*).tw. (38749) |
| 57 (hydrogen adj4 carbonate*).tw. (609) |
| 58 (carbonic adj4 acid adj4 ion*).tw. (29) |
| 59 (potassium or KCL or K39 or Kalium).tw. (178435) |
| 60 (phosphate* or orthophosphate*).tw. (286237) |
| 61 or/22-60 (5962035) |
| 62 21 and 61 (16102) |
| 63 nonhuman/ not human/ (4552889) |
| 64 62 not 63 (14879) |
| 65 limit 64 to english language (13389) |
| 66 limit 65 to dc=20140601-20200213 (5832) |
| 67 limit 66 to (conference abstract or conference paper or “conference review”) (2751) |
| 68 66 not 67 (3081) |
| 69 exp Health Economics/ (827289) |
| 70 exp “Health Care Cost”/ (285058) |
| 71 exp Pharmacoeconomics/ (199133) |
| 72 Monte Carlo Method/ (39076) |
| 73 Decision Tree/ (12245) |
| 74 econom$.tw. (355008) |
| 75 cba.tw. (12591) |
| 76 cea.tw. (33884) |
| 77 cua.tw. (1450) |
| 78 markov$.tw. (29397) |
| 79 (monte adj carlo).tw. (46984) |
| 80 (decision adj3 (tree$ or analys$)).tw. (22336) |
| 81 (cost or costs or costing$ or costly or costed).tw. (745459) |
| 82 (price$ or pricing$).tw. (55614) |
| 83 budget$.tw. (37516) |
| 84 expenditure$.tw. (72538) |
| 85 (value adj3 (money or monetary)).tw. (3356) |
| 86 (pharmacoeconomic$ or (pharmaco adj economic$)).tw. (8505) |
| 87 or/69-86 (1707223) |
| 88 “Quality of Life”/ (452733) |
| 89 Quality Adjusted Life Year/ (25689) |
| 90 Quality of Life Index/ (2721) |
| 91 Short Form 36/ (27717) |
| 92 Health Status/ (124395) |
| 93 quality of life.tw. (421848) |
| 94 quality adjusted life.tw. (18980) |
| 95 (qaly$ or qald$ or qale$ or qtime$).tw. (19446) |
| 96 disability adjusted life.tw. (3853) |
| 97 daly$.tw. (3789) |
| 98 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. (40321) |
| 99 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. (2342) |
| 100 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. (9091) |
| 101 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. (57) |
| 102 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. (441) |
| 103 (euroqol or euro qol or eq5d or eq 5d).tw. (19515) |
| 104 (qol or hql or hqol or hrqol).tw. (92947) |
| 105 (hye or hyes).tw. (131) |
| 106 health$ year$ equivalent$.tw. (41) |
| 107 utilit$.tw. (279405) |
| 108 (hui or hui1 or hui2 or hui3).tw. (2197) |
| 109 disutili$.tw. (897) |
| 110 rosser.tw. (119) |
| 111 quality of wellbeing.tw. (42) |
| 112 quality of well-being.tw. (469) |
| 113 qwb.tw. (244) |
| 114 willingness to pay.tw. (8399) |
| 115 standard gamble$.tw. (1090) |
| 116 time trade off.tw. (1672) |
| 117 time tradeoff.tw. (288) |
| 118 tto.tw. (1619) |
| 119 or/88-118 (954177) |
| 120 87 or 119 (2509791) |
| 121 68 and 120 (286) |
| Database: Econlit |
|---|
| Strategy used: |
| Database: Econlit <1886 to January 30, 2020> |
| Search Strategy: |
| ------------------------------------------------------------ |
| 1 [Diabetic Ketoacidosis/] (0) |
| 2 (DK or DKA).tw. (18) |
| 3 (DM adj4 (keto* or acidi* or gastropare*)).tw. (1) |
| 4 or/1-3 (19) |
| 5 [exp Diabetes Mellitus/] (0) |
| 6 diabet*.tw. (584) |
| 7 (DM adj4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)).tw. (1) |
| 8 lada.tw. (0) |
| 9 (dm1 or iddm or t1d* or dka).tw. (10) |
| 10 (dm2 or t2d* or mody or niddm).tw. (46) |
| 11 (DM adj4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II)).tw. (1) |
| 12 (DM adj4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*)).tw. (0) |
| 13 (DM adj4 onset* adj4 (maturit* or adult* or slow*)).tw. (0) |
| 14 (DM adj4 depend* adj4 (non-insulin* or non insulin* or noninsulin*)).tw. (0) |
| 15 (DM adj4 (earl* or sudden onset or juvenile or child*)).tw. (1) |
| 16 or/5-15 (619) |
| 17 [Ketosis/] (0) |
| 18 (keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*).tw. (4) |
| 19 17 or 18 (4) |
| 20 16 and 19 (2) |
| 21 4 or 20 (21) |
| 22 [exp Fluid Therapy/] (0) |
| 23 [Rehydration Solutions/] (0) |
| 24 [Water-Electrolyte Balance/] (0) |
| 25 [Water-Electrolyte Imbalance/] (0) |
| 26 (fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*).tw. (34188) |
| 27 [Drug Administration Routes/] (0) |
| 28 (drug adj4 admin* adj4 route*).tw. (1) |
| 29 (drug adj4 deliver* adj4 system*).tw. (2) |
| 30 [Administration, Oral/] (0) |
| 31 [Administration, Intravenous/] (0) |
| 32 (oral* or intravenous or IV).tw. (6049) |
| 33 ((vein or venous) adj4 (infus* or inject* or drip or transfus*)).tw. (0) |
| 34 [Infusions, Intravenous/] (0) |
| 35 [Infusions, Intraosseous/] (0) |
| 36 ((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) adj4 (infus* or inject* or admin* or appl*)).tw. (3098) |
| 37 infusor*.tw. (0) |
| 38 (perfusion adj4 pump*).tw. (0) |
| 39 [exp Infusions, Subcutaneous/] (0) |
| 40 hypodermoclysis.tw. (0) |
| 41 [Infusion Pump/] (0) |
| 42 [Intubation, Gastrointestinal/] (0) |
| 43 (intubat* adj4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)).tw. (0) |
| 44 (fluid bolus or two bag or ORT).tw. (19) |
| 45 [Time Factors/] (0) |
| 46 (time adj4 factor*).tw. (1623) |
| 47 [Drug Administration Schedule/] (0) |
| 48 (drug adj4 admin* adj4 schedul*).tw. (0) |
| 49 (drug adj4 deliver* adj4 schedul*).tw. (0) |
| 50 [Sodium/] (0) |
| 51 (sodium* or salt*).tw. (653) |
| 52 (acetic adj4 acid).tw. (5) |
| 53 [exp Chlorides/] (0) |
| 54 (chloride* or chlorhydrate* or hydrochloride* or monochloride*).tw. (22) |
| 55 [Glucose/ or Glucose Solution, Hypertonic/] (0) |
| 56 (glucose or d-glucose or dextrose or l-glucose).tw. (47) |
| 57 [Saline Solution, Hypertonic/] (0) |
| 58 [Saline Solution/ or Ringer’s Lactate/] (0) |
| 59 (saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*).tw. (888) |
| 60 [exp Bicarbonates/] (0) |
| 61 (bicarbonate* or dicarbonate* or baros* or hydrocarbonate*).tw. (3) |
| 62 (hydrogen adj4 carbonate*).tw. (0) |
| 63 (carbonic adj4 acid adj4 ion*).tw. (0) |
| 64 [Potassium/ or Potassium Acetate/] (0) |
| 65 (potassium or KCL or K39 or Kalium).tw. (46) |
| 66 [Phosphates/] (0) |
| 67 (phosphate* or orthophosphate*).tw. (106) |
| 68 or/22-67 (46140) |
| 69 21 and 68 (2) |
| 70 [animals/ not humans/] (0) |
| 71 69 not 70 (2) |
| 72 limit 71 to english language [Limit not valid; records were retained] (2) |
| Database: CRD - NHS EED and HTA |
|---|
| Strategy used: |
| Line Search Hits |
| 1 (MeSH DESCRIPTOR Diabetic Ketoacidosis) 12 Delete |
| 2 (((DK or DKA))) 520 Delete |
| 3 ((DM) AND ((keto* or acidi* or gastropare*))) 3 Delete |
| 4 (#1 OR #2 OR #3) 532 Delete |
| 5 (MeSH DESCRIPTOR Diabetes Mellitus EXPLODE ALL TREES) 2444 Delete |
| 6 (diabet*) 4478 Delete |
| 7 ((DM) AND (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)) 29 Delete |
| 8 (lada) 1 Delete |
| 9 (dm1 or iddm or t1d* or dka) 53 Delete |
| 10 (dm2 or t2d* or mody or niddm) 83 Delete |
| 11 ((DM) AND ((“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II))) 53 Delete |
| 12 ((DM) AND ((autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*))) 8 Delete |
| 13 ((DM) AND (onset*) AND ((maturit* or adult* or slow*))) 14 Delete |
| 14 ((DM) AND (depend*) AND ((non-insulin* or non insulin* or noninsulin*))) 4 Delete |
| 15 ((DM) AND ((earl* or sudden onset or juvenile or child*))) 118 Delete |
| 16 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) 4631 Delete |
| 17 (MeSH DESCRIPTOR Ketosis) 3 Delete |
| 18 (((keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*))) 324 Delete |
| 19 (#17 OR #18) 324 Delete |
| 20 (#16 AND #19) 70 Delete |
| 21 (#4 OR #20) 587 Delete |
| 22 (MeSH DESCRIPTOR Fluid Therapy EXPLODE ALL TREES) 131 Delete |
| 23 (MeSH DESCRIPTOR Rehydration Solutions) 19 Delete |
| 24 (MeSH DESCRIPTOR Water-Electrolyte Balance) 5 Delete |
| 25 (MeSH DESCRIPTOR Water-Electrolyte Imbalance) 1 Delete |
| 26 (((fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*))) 2135 Delete |
| 27 (MeSH DESCRIPTOR Drug Administration Routes) 35 Delete |
| 28 (((drug and admin* and route*))) 311 Delete |
| 29 (((drug and deliver* and system*))) 1108 Delete |
| 30 (MeSH DESCRIPTOR Administration, Oral) 726 Delete |
| 31 (MeSH DESCRIPTOR Administration, Intravenous) 52 Delete |
| 32 (((oral* or intravenous or IV))) 7863 Delete |
| 33 (((vein or venous)) AND ((infus* or inject* or drip or transfus*))) 309 Delete |
| 34 (MeSH DESCRIPTOR Infusions, Intravenous) 351 Delete |
| 35 (MeSH DESCRIPTOR Infusions, Intraosseous) 3 Delete |
| 36 (((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip)) AND ((infus* or inject* or admin* or appl*))) 18089 Delete |
| 37 (infusor*) 4 Delete |
| 38 (perfusion and pump*) 14 Delete |
| 39 (MeSH DESCRIPTOR Infusions, Subcutaneous EXPLODE ALL TREES) 21 Delete |
| 40 (hypodermoclysis) 2 Delete |
| 41 (MeSH DESCRIPTOR Infusion Pumps) 43 Delete |
| 42 (MeSH DESCRIPTOR Intubation, Gastrointestinal) 57 Delete |
| 43 ((intubat*) AND (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)) 77 Delete |
| 44 (fluid bolus or two bag or ORT) 14 Delete |
| 45 (MeSH DESCRIPTOR Time Factors) 3076 Delete |
| 46 (time and factor*) 7112 Delete |
| 47 (MeSH DESCRIPTOR Drug Administration Schedule) 815 Delete |
| 48 (drug and admin* and schedul*) 1218 Delete |
| 49 (drug and deliver* and schedul*) 134 Delete |
| 50 (MeSH DESCRIPTOR Sodium) 13 Delete |
| 51 (sodium* or salt*) 894 Delete |
| 52 (acetic and acid) 42 Delete |
| 53 (MeSH DESCRIPTOR Chlorides EXPLODE ALL TREES) 69 Delete |
| 54 (((chloride* or chlorhydrate* or hydrochloride* or monochloride*))) 602 Delete |
| 55 (MeSH DESCRIPTOR Glucose) 56 Delete |
| 56 (MeSH DESCRIPTOR Glucose Solution, Hypertonic) 2 Delete |
| 57 (((glucose or d-glucose or dextrose or l-glucose))) 1283 Delete |
| 58 (MeSH DESCRIPTOR Saline Solution, Hypertonic) 24 Delete |
| 59 (((saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*))) 621 Delete |
| 60 (MeSH DESCRIPTOR Bicarbonates EXPLODE ALL TREES) 22 Delete |
| 61 (((bicarbonate* or dicarbonate* or baros* or hydrocarbonate*))) 73 Delete |
| 62 (((hydrogen and carbonate*))) 0 Delete |
| 63 (((carbonic and acid and ion*))) 0 Delete |
| 64 (MeSH DESCRIPTOR Potassium) 23 Delete |
| 65 (MeSH DESCRIPTOR Potassium Acetate) 0 Delete |
| 66 (((potassium or KCL or K39 or Kalium))) 205 Delete |
| 67 (MeSH DESCRIPTOR Phosphates) 32 Delete |
| 68 (((phosphate* or orthophosphate*))) 189 Delete |
| 69 (#22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68) 28822 Delete |
| 70 (#21 AND #69) 217 Delete |
| 71 ((#70) WHERE LPD FROM 01/06/2014 TO 12/02/2020) 6 Delete |
| Database: Ovid Emcare |
|---|
| Strategy used: |
| Database: Ovid Emcare <1995 to 2020 week 06> |
| Search Strategy: |
| ------------------------------------------------------------ |
| 1 Diabetic Ketoacidosis/ (3417) |
| 2 (DK or DKA).tw. (1250) |
| 3 (DM adj4 (keto* or acidi* or gastropare*)).tw. (12) |
| 4 or/1-3 (3986) |
| 5 exp Diabetes Mellitus/ (215456) |
| 6 diabet*.tw. (184022) |
| 7 (DM adj4 (“type 1” or type1 or “type I” or “type one” or T1 or T-1 or TI or T-I)).tw. (502) |
| 8 lada.tw. (177) |
| 9 (dm1 or iddm or t1d* or dka).tw. (5731) |
| 10 (dm2 or t2d* or mody or niddm).tw. (12425) |
| 11 (DM adj4 (“type 2” or type2 or “type ii” or “type two” or T2 or T-2 or TII or T-II)).tw. (1652) |
| 12 (DM adj4 (autoimmun* or auto immun* or brittle or labile or insulin depend* or insulin deficien*)).tw. (78) |
| 13 (DM adj4 onset* adj4 (maturit* or adult* or slow*)).tw. (10) |
| 14 (DM adj4 depend* adj4 (non-insulin* or non insulin* or noninsulin*)).tw. (29) |
| 15 (DM adj4 (earl* or sudden onset or juvenile or child*)).tw. (307) |
| 16 or/5-15 (240388) |
| 17 Ketoacidosis/ (1266) |
| 18 (keto* or acidos* or acidoketos* or ketoacidaemi* or ketoacidemi* or hyperketo* or hyper-keto* or ketotic or ketonuri* or keton?emi* or acetonemi* or acetonuri*).tw. (16402) |
| 19 17 or 18 (16875) |
| 20 16 and 19 (3897) |
| 21 4 or 20 (5854) |
| 22 exp Fluid Therapy/ or exp Infusion fluid/ (37230) |
| 23 oral rehydration solution/ (954) |
| 24 exp electrolyte balance/ or exp electrolyte/ (46925) |
| 25 (fluid* or solution* or electrolyte* or hydrat* or rehydrat* or re-hydrat* or “re hydrat*” or resuscitat*).tw. (221493) |
| 26 exp Drug Administration Route/ (86865) |
| 27 (drug adj4 admin* adj4 route*).tw. (328) |
| 28 (drug adj4 deliver* adj4 system*).tw. (3222) |
| 29 Oral Drug Administration/ (21771) |
| 30 exp Intravenous Drug Administration/ (27102) |
| 31 (oral* or intravenous or IV).tw. (290648) |
| 32 ((vein or venous) adj4 (infus* or inject* or drip or transfus*)).tw. (2146) |
| 33 exp Intraosseous Drug Administration/ (254) |
| 34 ((intra-osseous or intraosseous or IO or intra-bone or intrabone or “intra bone” or system* or pump* or subcutan* or drip) adj4 (infus* or inject* or admin* or appl*)).tw. (32164) |
| 35 infusor*.tw. (62) |
| 36 (perfusion adj4 pump*).tw. (123) |
| 37 Subcutaneous Drug Administration/ or Hypodermoclysis/ (5703) |
| 38 hypodermoclys*.tw. (69) |
| 39 exp Infusion Pump/ (2611) |
| 40 exp Digestive Tract Intubation/ (1699) |
| 41 (intubat* adj4 (gastrointestin* or gastro-intestin* or “gastro intestin*” or nasogastric*)).tw. (155) |
| 42 (fluid bolus or two bag or ORT).tw. (676) |
| 43 Time Factor/ (1165) |
| 44 (time adj4 factor*).tw. (7041) |
| 45 exp Drug Administration/ (97925) |
| 46 (drug adj4 admin* adj4 schedul*).tw. (94) |
| 47 (drug adj4 deliver* adj4 schedul*).tw. (11) |
| 48 Acetic Acid/ or exp Inorganic Salt/ (124456) |
| 49 (sodium* or salt*).tw. (52046) |
| 50 (acetic adj4 acid).tw. (3522) |
| 51 Chloride/ (3547) |
| 52 (chloride* or chlorhydrate* or hydrochloride* or monochloride*).tw. (16131) |
| 53 Glucose/ (81096) |
| 54 (glucose or d-glucose or dextrose or l-glucose).tw. (88868) |
| 55 (saline* or Na-CI* or Na-Cl* or Nacl* or NacI* or hartmann* or ringer*).tw. (43420) |
| 56 (bicarbonate* or dicarbonate* or baros* or hydrocarbonate*).tw. (3899) |
| 57 (hydrogen adj4 carbonate*).tw. (39) |
| 58 (carbonic adj4 acid adj4 ion*).tw. (1) |
| 59 (potassium or KCL or K39 or Kalium).tw. (14715) |
| 60 (phosphate* or orthophosphate*).tw. (24140) |
| 61 or/22-60 (828600) |
| 62 21 and 61 (3250) |
| 63 nonhuman/ not human/ (392209) |
| 64 62 not 63 (3135) |
| 65 limit 64 to english language (2973) |
| 66 limit 65 to dc=20140601-20200212 (1301) |
| 67 limit 66 to (conference abstract or conference paper or “conference review”) (31) |
| 68 66 not 67 (1270) |
| 69 exp Health Economics/ (285451) |
| 70 exp “Health Care Cost”/ (121118) |
| 71 exp Pharmacoeconomics/ (52835) |
| 72 Monte Carlo Method/ (9058) |
| 73 Decision Tree/ (2689) |
| 74 econom$.tw. (102945) |
| 75 cba.tw. (881) |
| 76 cea.tw. (3286) |
| 77 cua.tw. (170) |
| 78 markov$.tw. (7017) |
| 79 (monte adj carlo).tw. (9378) |
| 80 (decision adj3 (tree$ or analys$)).tw. (6611) |
| 81 (cost or costs or costing$ or costly or costed).tw. (198934) |
| 82 (price$ or pricing$).tw. (16658) |
| 83 budget$.tw. (11439) |
| 84 expenditure$.tw. (25385) |
| 85 (value adj3 (money or monetary)).tw. (1354) |
| 86 (pharmacoeconomic$ or (pharmaco adj economic$)).tw. (3314) |
| 87 or/69-86 (491128) |
| 88 “Quality of Life”/ (150771) |
| 89 Quality Adjusted Life Year/ (9780) |
| 90 Quality of Life Index/ (1214) |
| 91 Short Form 36/ (11756) |
| 92 Health Status/ (53021) |
| 93 quality of life.tw. (122237) |
| 94 quality adjusted life.tw. (6735) |
| 95 (qaly$ or qald$ or qale$ or qtime$).tw. (5629) |
| 96 disability adjusted life.tw. (1429) |
| 97 daly$.tw. (1230) |
| 98 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. (13125) |
| 99 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. (262) |
| 100 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. (3309) |
| 101 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. (9) |
| 102 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. (111) |
| 103 (euroqol or euro qol or eq5d or eq 5d).tw. (5810) |
| 104 (qol or hql or hqol or hrqol).tw. (23761) |
| 105 (hye or hyes).tw. (53) |
| 106 health$ year$ equivalent$.tw. (24) |
| 107 utilit$.tw. (62991) |
| 108 (hui or hui1 or hui2 or hui3).tw. (741) |
| 109 disutili$.tw. (277) |
| 110 rosser.tw. (48) |
| 111 quality of wellbeing.tw. (19) |
| 112 quality of well-being.tw. (250) |
| 113 qwb.tw. (131) |
| 114 willingness to pay.tw. (2971) |
| 115 standard gamble$.tw. (501) |
| 116 time trade off.tw. (684) |
| 117 time tradeoff.tw. (150) |
| 118 tto.tw. (538) |
| 119 or/88-118 (285208) |
| 120 87 or 119 (723040) |
| 121 68 and 120 (139) |
Appendix E. Effectiveness evidence
Type of fluid – IV fluids
Observational studies
Basnet 2014 (PDF, 176K)
Bergmann 2018 (PDF, 178K)
Savaş-Erdeve 2011 (PDF, 186K)
Type of fluid and rate of rehydration
RCTs
Kuppermann 2018 (PDF, 205K)
IV fluids + Additives
Volume of rehydration
RCTs
Bakes 2016 (PDF, 186K)
Appendix F. Forest plots
Type of fluid – IV fluids
Moderate to severe DKA
0.9% Saline vs Hartmann’s solution as initial IV fluid
0.9% saline vs hypertonic saline (3% NaCl) as initial IV fluid
Outcomes during 1 hour of treatment
Outcomes during 12 hours of treatment
All severities of DKA
0.9% saline vs Plasma-Lyte-A as initial IV fluid
Outcomes during 24 hours of treatment
Outcomes during 48 hours of treatment
0.9% saline vs 0.45% saline for replacement of deficit
Outcomes during treatment of DKA
Confirmed decline in Glasgow Coma Scale score to <14
Outcomes 2 to 6 months after hospitalisation
0.9% saline vs 0.45% saline as post-bolus re-hydration fluid
Normal saline vs Ringer’s lactate
Type 1 diabetes - All severities of DKA
75 mEq/L NaCl vs 100 mEq/L NaCl after initial rehydration
Outcomes during 1 hour of treatment
Outcomes during 24 hours of treatment
IV + Additives
Severe DKA
IV fluid + sodium bicarbonate vs IV fluid+ no sodium bicarbonate
All severities of DKA
IV fluid (Lactate Ringers or Lactate Ringers with saline) with sodium bicarbonate vs IV fluid (Lactate Ringers or Lactate Ringers with saline) alone
Rate of rehydration
All severities of DKA
Fast rate vs slow rate for the replacement of deficit
Outcomes during treatment of DKA
Confirmed decline in Glasgow Coma Scale score to <14
Outcomes 2 to 6 months after hospitalisation
Type 1 diabetes- All severities of DKA
Rapid rate vs slower rate
Outcomes during the treatment of DKA
Fast rate vs slow rate
Outcomes during the treatment of DKA
Time in which acidosis resolved (hours)
Volume
Type 1 diabetes- All severities of DKA
High volume vs low volume
Outcomes during treatment of DKA
Appendix G. Additional data
Type of fluids
Moderate to severe DKA
0.9% Saline vs Hartmann’s solution as initial IV fluid
0.9% Saline vs Hypertonic saline (3% NaCl) as initial IV fluid
Outcomes during treatment of DKA
| Study name | 0.9% saline | Hypertonic saline | P value* | Notes |
|---|---|---|---|---|
| Average time needed for the correction of hyperglycaemia (hours) |
Risk of bias: Serious1 Directness: No serious | |||
| Shafi 2018 | 7.13 | 7.15 | 0.974 | |
| Time needed for the resolution of acidosis (hours) | ||||
| Shafi 2018 | 17.35 | 18 | 0.782 | |
Data presented as mean (SD not reported)
- 1
Baseline differences (e.g. age, sex, type of diabetes) between arms not reported.
- *
Used Mann-Whitney U test. P-value of 0.05 was considered to indicate statistical significance.
All severities of DKA
0.9% Saline vs Plasma-Lyte-A as initial IV fluid
Outcomes during treatment of DKA
| Study name | 0.9% saline | Plasma-lyte-A | P value* | Notes |
|---|---|---|---|---|
| Length of intensive care unit (ICU) stay (hours) |
Risk of bias: Serious1 Directness: No serious | |||
| Williams 2020 | 48 (48, 60) | 47 (24, 54) | 0.276 | |
| Length of hospital stay (days) | ||||
| Williams 2020 | 9 (8, 12) | 10.0 (8.25, 11) | 0.396 | |
Data presented as median (interquartile range)
- 1
There was a significant difference in the number of participants with new onset diabetes in the two arms of the trial
- *
Used unpaired student’s t-test or the Wilcoxon rank-sum test. P-value (two-tailed)<0.05 was taken as significant.
0.9% Saline vs 0.45% saline for replacement of deficit
Outcomes during treatment of DKA
| Study name | 0.9% saline | 0.45% saline | P value* | Notes |
|---|---|---|---|---|
| Time to resolution of DKA (hours)– fast rate | ||||
| Kuppermann 2018 | 14.0 (9.8-18.3) | 14.0 (10.2-18.3) | 0.48 |
Risk of bias: No serious Directness: No serious |
| Time to resolution of DKA (hours)– slow rate | ||||
| Kuppermann 2018 | 13.6 (10.0-18.5) | 14.9 (9.8-18.6) | 0.48 |
Risk of bias: No serious Directness: No serious |
| Time to hospital discharge (hours)- fast rate | ||||
| Kuppermann 2018 | 47.4 (26.6-67.2) | 46.3 (27.3-66.3) | 0.71 |
Risk of bias: No serious Directness: No serious |
| Time to hospital discharge (hours)- slow rate | ||||
| Kuppermann 2018 | 46.4 (27.2-69.0) | 48.6 (28.0-68.8) | 0.71 |
Risk of bias: No serious Directness: No serious |
Data presented as median (25th and 75th percentile)
- *
p values are from a Van Elteren test
Normal saline vs Ringer’s lactate
Outcomes during treatment of DKA
| Study name | Normal saline | Ringer’s lactate | P value | Notes |
|---|---|---|---|---|
| Length of hospital stay (days) | ||||
| Bergmann 2018 | 2 (1-3) | 2 (1-3) | Not reported |
Risk of bias: Serious1 Directness: Serious2 |
Data presented as median and interquartile range.
- 1
DKA protocol (e.g. co-interventions) were not described in the study
- 2
Definition of DKA not provided.
Rate of rehydration
All severities of DKA
0.9% Saline vs 0.45% saline for replacement of deficit
Outcomes during treatment of DKA
| Study name | Fast rate | Slow rate | P value* | Notes |
|---|---|---|---|---|
| Time to resolution of DKA (hours)– 0.45% saline | ||||
| Kuppermann 2018 | 14.0 (10.2 - 18.3) | 14.9 (9.9-18.6) | 0.28 |
Risk of bias: No serious Directness: No serious |
| Time to resolution of DKA (hours)– 0.9% saline | ||||
| Kuppermann 2018 | 14.0 (9.8-18.3) | 13.6 (10.0-18.5) | 0.28 |
Risk of bias: No serious Directness: No serious |
| Time to hospital discharge (hours)- 0.45% saline | ||||
| Kuppermann 2018 | 46.3 (27.3-66.3) | 48.6 (28.0-68.8) | 0.34 |
Risk of bias: No serious Directness: No serious |
| Time to hospital discharge (hours)- 0.9% saline | ||||
| Kuppermann 2018 | 47.4 (26.6-67.2) | 46.4 (27.2-69.0) | 0.34 |
Risk of bias: No serious Directness: No serious |
Data presented as median (25th and 75th percentile)
- *
p values are from a Van Elteren test
Appendix H. GRADE tables
Type of fluid – IV fluids
Moderate to severe DKA
0.9% Saline vs Hartmann’s solution as initial IV fluid
0.9% Saline vs hypertonic saline (3% NaCl) as initial IV fluid
All severities of DKA
0.9% Saline vs Plasma-Lyte-A as initial IV fluid
Outcomes during 24 hours of treatment
0.9% Saline vs 0.45% saline for replacement of deficit
0.9% Saline vs 0.45% saline post-bolus re-hydration fluid
Normal saline vs Ringer’s lactate
Type 1 diabetes - All severities of DKA
75 mEq/L NaCl vs 100 mEq/L NaCl after initial rehydration
IV + Additives
Severe DKA
IV fluid (not specified) with sodium bicarbonate vs IV fluid (not specified) with no sodium bicarbonate
All severities of DKA
IV fluid (Lactate Ringers or Lactate Ringers with saline) with sodium bicarbonate vs IV fluid (Lactate Ringers or Lactate Ringers with saline) alone
Rate of rehydration
All severities of DKA
Fast rate vs slow rate for the replacement of deficit
Type 1 diabetes- All severities of DKA
Rapid rate vs slower rate
Fast rate vs slow rate
Volume of rehydration
Type 1 diabetes- All severities of DKA
High volume vs low volume
Appendix J. Economic evidence tables
None
Appendix K. Health economic model
This question was not prioritised for health economic modelling.
The cost of managing an episode of DKA is very high when compared to the price of the fluids. As a result, the effectiveness of the fluids will determine the most cost-effective option.
Appendix L. Excluded studies
RCTs
| Study | Code [Reason] |
|---|---|
| Antequera Martín, AM, Barea Mendoza, JA, Muriel, A et al (2019) Buffered solutions versus 0.9% saline for resuscitation in critically ill adults and children. Cochrane Database of Systematic Reviews [PMC free article: PMC6647932] [PubMed: 31334842] | - Systematic review does not include population of interest |
| Carcillo, Joseph A (2014) Intravenous fluid choices in critically ill children. Current opinion in critical care 20(4): 396–401 [PubMed: 24979714] | - Review article but not a systematic review |
| Dhochak, N; Jayashree, M; Singhi, S (2018) A randomized controlled trial of one bag vs. two bag system of fluid delivery in children with diabetic ketoacidosis: Experience from a developing country. Journal of critical care 43: 340–345 [PubMed: 29066219] | - Study does not contain a relevant intervention [Study compared one bag system (IV fluids with desired dextrose) with two bag system (first bag with no dextrose and second bag with dextrose).] |
| Glaser, Nicole and Kuppermann, Nathan (2019) Fluid treatment for children with diabetic ketoacidosis: How do the results of the pediatric emergency care applied research network Fluid Therapies Under Investigation in Diabetic Ketoacidosis (FLUID) Trial change our perspective? Pediatric diabetes 20(1): 10–14 [PubMed: 30417497] | - Review article but not a systematic review [Review of PECARN trial] |
| Koves, Ildiko H, Leu, Michael G, Spencer, Suzanne et al (2014) Improving care for pediatric diabetic ketoacidosis. Pediatrics 134(3): e848–56 [PubMed: 25092935] | - Comparator in study does not match that specified in protocol [Cohort study with a historical control. The control arm represented treatment before the implementation of a DKA protocol. Before the protocol there was no consistent treatment of DKA.] |
| Usman, A., Bakry, M.M., Mustafa, N. et al (2019) Correlation of acidosis-adjusted potassium level and cardiovascular outcomes in diabetic ketoacidosis: A systematic review. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 12: 1323–1338 [PMC free article: PMC6689561] [PubMed: 31496770] | - Systematic review does not include population of interest |
Observational studies
Studies highlighted in bold were included in the previous (2015) update.
| Study | Code [Reason] |
|---|---|
| Becker DJ, Brown DR, Steranka BH et al (1983) Phosphate replacement during treatment of diabetic ketosis. Effects on calcium and phosphorus homeostasis. American journal of diseases of children (1960) 137(3): 241–246 [PubMed: 6401918] | - Comparator in study does not match that specified in protocol [Control group consisted of children who were neither acidotic nor clinically dehydrated on admission and were treated with subcutaneous crystalline insulin, oral fluids and a diabetic diet without potassium or phosphorus supplements] |
| Edge JA, Jakes RW, Roy Y et al (2006) The UK case-control study of cerebral oedema complicating diabetic ketoacidosis in children. Diabetologia 49(9): 2002–2009 [PubMed: 16847700] |
- Not a relevant study design [Case control study] - Study does not contain a relevant intervention [Cases defined as patients with cerebral oedema and control as those without cerebral oedema] |
| Flood, Kayla, Nour, Munier, Holt, Tanya et al (2019) Implementation and Evaluation of a Diabetic Ketoacidosis Order Set in Pediatric Type 1 Diabetes at a Tertiary Care Hospital: A Quality-Improvement Initiative. Canadian journal of diabetes 43(5): 297–303 [PubMed: 30777707] |
- Study does not contain a relevant intervention [Study assessed update of a new DKA protocol (details of new protocol not provided)] |
| Glaser N, Barnett P, McCaslin I et al (2001) Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. The New England journal of medicine 344(4): 264–269 [PubMed: 11172153] |
- Not a relevant study design [Case control study] |
| Hsia, Daniel S, Tarai, Sarah G, Alimi, Amir et al (2015) Fluid management in pediatric patients with DKA and rates of suspected clinical cerebral edema. Pediatric diabetes 16(5): 338–44 [PMC free article: PMC4496255] [PubMed: 25800410] |
- Study does not contain a relevant intervention [Study compared two different protocols that differed in type of fluid, rate and additives.] |
| Lawrence SE, Cummings EA, Gaboury I et al (2005) Population-based study of incidence and risk factors for cerebral edema in pediatric diabetic ketoacidosis. The Journal of pediatrics 146(5): 688–692 [PubMed: 15870676] |
- Not a relevant study design [Case control study] |
| Mahoney CP; Vlcek BW; DelAguila M (1999) Risk factors for developing brain herniation during diabetic ketoacidosis. Pediatric neurology 21(4): 721–727 [PubMed: 10580884] |
- Not a relevant study design [Chart review] |
| Munir, I., Fargo, R., Garrison, R. et al (2017) Comparison of a ‘two-bag system’ versus conventional treatment protocol (‘one-bag system’) in the management of diabetic ketoacidosis. BMJ Open Diabetes Research and Care 5(1): e000395 [PMC free article: PMC5574429] [PubMed: 28878933] | - Wrong population [Study included adults] |
| Pruitt, L.G., Jones, G., Musso, M. et al (2019) Intravenous fluid bolus rates and pediatric diabetic ketoacidosis resolution. American Journal of Emergency Medicine 37(12): 2239–2241 [PubMed: 30967324] |
- Not a relevant study design [Retrospective chart review] |
| Ronsley, Rebecca, Islam, Nazrul, Ronsley, Claire et al (2018) Adherence to a pediatric diabetic ketoacidosis protocol in children presenting to a tertiary care hospital. Pediatric diabetes 19(2): 333–338 [PubMed: 28664545] |
- Study does not contain a relevant intervention [Study examined the adherence of a DKA protocol] |
| Velasco Md, Jacqueline P, Fogel PhD, Joshua, Levine Md PhD, Robert L et al (2017) Potential Clinical Benefits of a Two-bag System for Fluid Management in Pediatric Intensive Care Unit Patients with Diabetic Ketoacidosis. Pediatric endocrinology, diabetes, and metabolism 23(1): 6–13 [PubMed: 29073302] |
- Study does not contain a relevant intervention [Compared one bag system (IV fluid with electrolytes used and new bag ordered with appropriate glucose content when first bag is depleted) with two bag system (two bags with identical electrolyte content but with different dextrose concentrations that are administered simultaneously).] |
| Veverka, Megan, Marsh, Kourtney, Norman, Susan et al (2016) A Pediatric Diabetic Ketoacidosis Management Protocol Incorporating a Two-Bag Intravenous Fluid System Decreases Duration of Intravenous Insulin Therapy. The journal of pediatric pharmacology and therapeutics: JPPT: the official journal of PPAG 21(6): 512–517 [PMC free article: PMC5178813] [PubMed: 28018153] |
- Comparator in study does not match that specified in protocol [Study compared people receiving two-bag protocol to those who did not receive protocol. It is unclear what intervention the control group received] |
Appendix M. Research recommendations – full details
M.1. Research recommendation
In children and young people with diabetic ketoacidosis, what is the most effective resuscitation fluid (0.9% sodium chloride vs PlasmaLyte 148) for managing of DKA?
Why this is important
Based on the available evidence, the committee recommended the use of 0.9% sodium chloride as resuscitation fluid in the management of DKA. However, the committee were aware that some paediatric units are using PlasmaLyte 148 for initial resuscitation. PlasmaLyte 148 has a lower sodium and chloride content compared to 0.9% sodium chloride and because of this, it is suggested that hyperchloremic acidosis is less likely to occur. However, research is required to demonstrate the effectiveness of PlasmaLyte 148 as resuscitation fluid in the management of DKA in children and young people.
Rationale for research recommendation
Only one study was identified which compared PlasmaLyte (study refers to fluid as PlasmaLyte-A) with 0.9% normal saline as initial fluid in the management of DKA in children. This study could not differentiate between the two fluids in outcomes such as incidence of acute kidney injury, mortality, and cerebral oedema. Due to the lack of evidence, the committee were unable to make recommendations but noted that further robust research is required to ascertain the effectiveness of PlasmaLyte 148 as resuscitation fluid in the management of DKA in children and young people.
Modified PICO table
| Population | Children and young people with type 1 or type 2 diabetes with diabetic ketoacidosis (although the diabetes may not yet have been recognised, for example, if the child or young person is presenting for the first time with DKA) |
|---|---|
| Interventions | PlasmaLyte 148 |
| Comparator | 0.9% sodium chloride |
| Outcomes |
|
| Study design | Randomised controlled trial |
| Additional information | Study should be adequately powered |
Final
Methods, evidence and recommendations
These evidence reviews were developed by the Guidelines Update Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Review Fluid and electrolyte therapy in childhood diabetic ketoacidosis management: A rationale for new national guideline.[Diabet Med. 2021]Review Fluid and electrolyte therapy in childhood diabetic ketoacidosis management: A rationale for new national guideline.Agwu JC, Ng SM. Diabet Med. 2021 Aug; 38(8):e14595. Epub 2021 May 17.
- Review Fluid Therapy For Pediatric Patients With Diabetic Ketoacidosis: Current Perspectives.[Diabetes Metab Syndr Obes. 2019]Review Fluid Therapy For Pediatric Patients With Diabetic Ketoacidosis: Current Perspectives.Jayashree M, Williams V, Iyer R. Diabetes Metab Syndr Obes. 2019; 12:2355-2361. Epub 2019 Nov 12.
- Review Evidence reviews for long-acting insulins in type 1 diabetes: Type 1 diabetes in adults: diagnosis and management: Evidence review A[ 2021]Review Evidence reviews for long-acting insulins in type 1 diabetes: Type 1 diabetes in adults: diagnosis and management: Evidence review A. 2021 Jul
- Implementation and Evaluation of a Diabetic Ketoacidosis Order Set in Pediatric Type 1 Diabetes at a Tertiary Care Hospital: A Quality-Improvement Initiative.[Can J Diabetes. 2019]Implementation and Evaluation of a Diabetic Ketoacidosis Order Set in Pediatric Type 1 Diabetes at a Tertiary Care Hospital: A Quality-Improvement Initiative.Flood K, Nour M, Holt T, Cattell V, Krochak C, Inman M. Can J Diabetes. 2019 Jul; 43(5):297-303. Epub 2018 Dec 26.
- Review Euglycemic diabetic ketoacidosis: A missed diagnosis.[World J Diabetes. 2021]Review Euglycemic diabetic ketoacidosis: A missed diagnosis.Nasa P, Chaudhary S, Shrivastava PK, Singh A. World J Diabetes. 2021 May 15; 12(5):514-523.
- Evidence reviews for fluid therapy for the management of diabetic ketoacidosisEvidence reviews for fluid therapy for the management of diabetic ketoacidosis
Your browsing activity is empty.
Activity recording is turned off.
See more...