NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Bladder Cancer: Diagnosis and Management. London: National Institute for Health and Care Excellence (NICE); 2015 Feb. (NICE Guideline, No. 2.)
5.1. Managing patients with distant metastases
5.1.1. First line chemotherapy
Review question: What is the optimal first-line chemotherapy regimen for patients with incurable locally advanced or metastatic bladder cancer?
Rationale
Most patients who die of bladder cancer will do so with metastatic disease. The main treatment used to prolong life and palliate/alleviate the symptoms is chemotherapy. Most studies report benefits in terms of response, symptom control and survival but this comes at the cost of significant treatment related toxicity. Though there are anecdotal reports of long term survivors these seem to be rare. Most clinicians use cisplatin based multiagent chemotherapy that is suitable for patients with normal renal function and good performance status. What evidence if there that the gains out way the toxicity? Does the treatment need to be cisplatin based or can less intensive therapy be used? Gemcitabine Cisplatin (GC) is widely used but is this best schedule in comparison to other schedules such as MVAC, CMV or accelerated MVAC. Does adding paclitaxel (GCP) improve results? Are there any other additional therapies that can be recommended? Carboplatin has a better toxicity profile (less sickness, fatigue, neuropathy but more myelosuppression) than cisplatin but there are concerns that carboplatin schedules such as gemcitabine carboplatin or carboplatin/methotrexate/vinblastine are less active. Does the evidence support this view leaving cisplatin based schedules as the treatment of choice despite their added toxicity? Most commonly 6 cycles of chemotherapy are used. Is there evidence that more or less chemotherapy than this would be suitable?
Many patients are elderly and/or have impaired performance status and/or impaired renal (kidney) function. In these patients there have been questions as to whether patients benefit from chemotherapy. Is the evidence that chemotherapy improves outcomes compared to best supportive care? If so what is the preferred schedule? Should carboplatin based treatment be used? Should some patients be treated with split dose cisplatin schedules? Are there are ‘platinum free’ schedules that are suitable? Are there groups or sub groups of patients that should/should not be treated?
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with incurable locally advanced or metastatic bladder cancer Cisplatin fit (GFR >60 PS 0/1) | Chemotherapy agents for first-line chemotherapy (alone or in combination): Methotrexate, Vinblastine, Adriamycin, Cisplatin, Gemcitabine, Carboplatin, Paclitaxel, Docetaxel | Each other (Cisplatin vs Non Cisplatin) No treatment |
METHODS
Information sources
A literature search was performed by the information specialist (EH).
Selection of studies
The information specialist (EH) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. The full articles were then obtained for potentially relevant studies and checked against the inclusion criteria. Randomised trials were selected for this review question.
Data synthesis
Data was extracted into RevMan and risk ratios were calculated when possible. Data from one systematic review of cisplatin versus non-cisplatin based chemotherapy was reported. Consideration was given to trials including patients eligible and not eligible for cisplatin-based chemotherapy.
RESULTS
Study quality and results
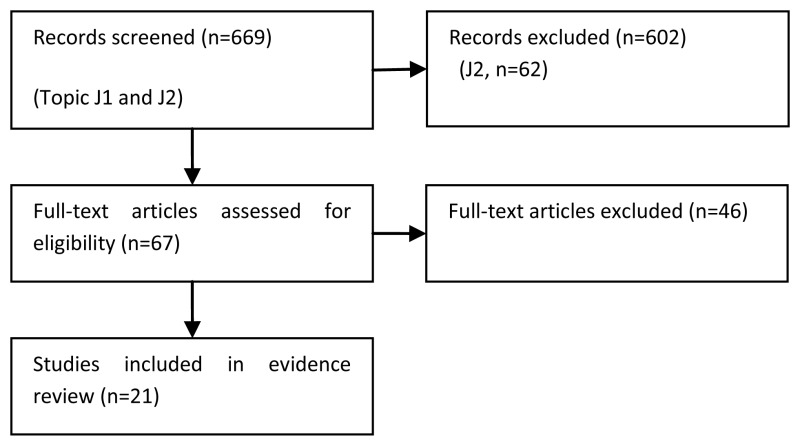
Evidence was identified from 21 randomised trials and is summarised in Tables 121-135.
Table 121
GRADE evidence profile: Cisplatin & Methotrexate (CM) versus Cisplatin (C).
Table 122
GRADE evidence profile: MVAC (Methotrexate, Vinblastine, Doxorubicin & Cisplatin) versus Methotrexate & Cisplatin (MC).
Table 123
GRADE evidence profile: CMV (Cisplatin, Methotrexate & Vinblastine) versus MV.
Table 124
GRADE evidence profile: MVAC (Methotrexate, Vinblastine, Doxorubicin & Cisplatin) versus Cisplatin.
Table 125
GRADE evidence profile: High-dose MVAC versus MVAC.
Table 126
GRADE evidence profile: Docetaxcel & Cisplatin (DC) with GCSF versus MVAC with GCSF.
Table 127
GRADE evidence profile: MVAC versus Gemcitabine & Cisplatin (GC).
Table 128
GRADE evidence profile: Dose dense MVAC (DD-MVAC) versus Dose dense Gemcitabine & Cisplatin (DD-GC).
Table 129
GRADE evidence profile: Gemcitabine & Cisplatin & Paclitaxel (PCG) versus Gemcitabine & Cisplatin (GC).
Table 130
GRADE evidence profile: MVAC versus Carboplatin & Paclitaxcel (CaP).
Table 131
GRADE evidence profile: Gemcitabine & Cisplatin (GC) versus Gemcitabine & Carboplatin (GCarbo).
Table 132
GRADE evidence profile: MVAC versus M-CAVI (Methotrexate, Carboplatin, Vinblastine).
Table 133
GRADE evidence profile: Cisplatin-based chemotherapy versus Carboplatin-based chemotherapy.
Table 134
GRADE evidence profile: Gemcitabine & Carboplatin (GCarbo) versus Methotrexate, Carboplatin & Vinblastine (M-CAVI) in patients unfit for cisplatin.
Evidence statements
Cisplatin-based chemotherapy
One phase II trial (Hillcoat et al., 1989) of 108 participants provided low quality evidence that there was no difference in overall survival between those treated with single agent Cisplatin (C) therapy or a combination of Cisplatin and Methotrexate (CM). Time to progression was longer with CM, but this difference was only significant during the first 12 months of therapy. Toxicity was greater in the CM arm, including haematological toxicity (26% vs. 7%) and mucositis (19% vs. 0%). Single agent Cisplatin was also compared to MVAC in one trial of 246 participants (Loehrer et al., 1992). Overall survival and progression-free survival were greater for MVAC than Cisplatin alone (low quality evidence). At 6-year follow-up, MVAC still showed a survival advantage over Cisplatin (Saxman et al., 1997). However, combined MVAC was more toxic than Cisplatin, with increased rates of grade 3-4 leukopenia, granulocytopenic fever, and mucositis. There were no differences in treatment-related mortality (4% vs. 0%). There was no evidence about health-related quality of life.
One trial (220 participants) of moderate quality reported increased duration of overall survival (14.2 months vs. 9.3 months) and time-to-progression (9.4 months vs. 6.1 months) with MVAC and granulocyte colony-stimulating factor (GCSF) compared to Docetaxel and Cisplatin with GCSF (Bamias et al., 2004). There were no differences in rates of grade 3-4 thrombocytopenia or anaemia. Neutropenia (36% vs. 19%) and neutropenic sepsis were more common in the MVAC arm. There were no differences in treatment-related mortality. One moderate quality trial (263 participants) compared high-dose intensity MVAC and GCSF (HD-MVAC) with classic MVAC (Sternberg et al., 2001/2006). After a median of 7.3 years follow-up, HD-MVAC produced a small improvement in risk of death and risk of progression. There were lower rates of whole blood cell toxicity and neutropenic fever with HD-MVAC, with no differences between arms for thrombocytopenia, mucositis and treatment-related mortality. Health-related quality of life was not reported.
One phase III trial (405 participants) of MVAC versus Gemcitabine and Cisplatin (GC) providing high quality evidence reported no differences in overall survival and progression-free survival between trial arms (von der Maase et al., 2000/2005). Rates of grade 3-4 anaemia and thrombocytopenia were greater in the GC arm, whereas neutropenia and neutropenic sepsis were more common in the MVAC arm. Mean quality of life scores were not reported but the authors state that quality of life (as measured by the EORTC QLQ C30) was maintained on both arms throughout the study with improvements in emotional functioning and pain. One observational study, where oncology professionals were interviewed as patient representatives, provided very low quality evidence that respondents were more likely to choose GC over MVAC for a reduced incidence of neutropenic sepsis, mucositis, or serious weight loss. Respondents were more willing to accept GC over MVAC even when a hypothetical life expectancy was reduced from 60 weeks to 45 weeks.
One randomised phase III trial (130 patients) of dose dense MVAC versus dose dense GC provided low quality evidence of no difference in overall survival or progression-free survival between groups. Grade 3-5 toxicities were reported in 50% of the DD-MVAC group and 44% of the DD-GC group. Two toxicity-related deaths were both in the DD-MVAC arm due to non-neutropenic sepsis (Bamias et al., 2013).
GC was compared with Pacitaxel, Gemcitabine and Cisplatin (PCG) in one randomised phase II trial of 85 patients (Lorusso et al., 2005) and one randomised phase III trial of 626 participants (Bellmunt et al., 2012). The phase III trial provided high quality evidence of no difference in overall survival and progression-free survival between trial arms. However, there was a small effect in the subgroup of patients with primary bladder tumours, with longer overall survival in patients treated with PCG (15.9 vs. 11.9 months, HR 0.80, 0.66 to 0.97). Grade 3-4 thrombocytopenia was more common in the GC arm, and grade 3-4 neutropenia was more common in the PCG arm (64% vs. 51%). Health-related quality of life was not reported.
Cisplatin-based versus carboplatin-based chemotherapy
Bellmunt et al. (1997) provided low quality evidence, comparing MVAC with methotrexate, carboplatin and vinblastine (M-CAVI) in 47 patients. Median disease-related survival was greater in the MVAC arm (hazard ratios were not reported). There were no differences in toxicity between arms. The study was terminated early and failed to reach accrual target. One underpowered trial (84 participants), which was closed early for slow accrual provided very low quality evidence comparing MVAC with carboplatin and paclitaxcel (CaP) (Dreicer et al., 2004). There were no differences between arms for overall survival and progression-free survival. Rates of neutropenia and anaemia were higher in the MVAC arm, but there were no differences in rates of thrombocytopenia and treatment-related mortality. It was reported that there were no differences in quality of life over time by treatment arm, but low numbers of participants were assessed for quality of life, which limits the precision of this outcome. One underpowered trial (110 participants) provided very low quality evidence of no difference in overall survival, time-to-progression, and toxicity between patients treated with Gemcitabine and Cisplatin versus Gemcitabine and Carboplatin (Dogliotti et al., 2007).
Four trials comparing cisplatin-based chemotherapy with carboplatin-based chemotherapy were included in the meta-analysis by Galsky et al. (2012). Very low quality evidence from two studies showed no difference in survival rate at 12 months (RR 0.76, 95% CI 0.56 to 1.07). Progression-free survival was not reported consistently across studies and could not be pooled in a meta-analysis. Therefore, overall tumour response rates and complete tumour response rates were pooled and risk ratios (95% CIs) were calculated. A partial tumour response was defined as a 50% reduction in bidimensional tumour measurements and a complete response as a resolution of radiographic abnormalities. A majority of patients had a performance status of 0 to 1 with adequate renal function. The meta-analysis demonstrated a higher likelihood of achieving an overall response (RR 1.34, 95% CI 1.04 to 1.71) and a complete response (RR 3.54, 95% CI 1.48 to 8.49) with cisplatin-based chemotherapy. However, this analysis is based on three small phase II studies and one phase III trial which was closed early due to poor accrual. The chemotherapy agents used and the doses of carboplatin used differed across studies.
Chemotherapy in ‘unfit’ patients
Moderate quality evidence for overall survival and progression-free survival was provided by one phase III RCT (238 participants) comparing Gemcitabine & Carboplatin (GCarbo) with Methotrexate & Carboplatin & Vinblastine (M-CAVI) (De Santis et al., 2012) in patients unfit for cisplatin-based therapy. After a median of 4.5 years follow-up there were no differences in overall survival (HR 0.94, 0.72 to 1.02) and progression-free survival (HR 1.04, 0.8 to 1.35) between the two treatments. GCarbo produced a lower rate of severe acute toxicity than M-CAVI (9% vs. 21%). There were no differences between treatments for changes in health-related quality of life from baseline to end of cycle 2, although mean scores were not reported and there was less than 50% response rate after the baseline assessment.
References to included studies
- Aristides M. Determining patient preferences for improved chemotoxicity during treatment for advanced bladder cancer. European Journal of Cancer Care. 2005;14(2):141–142. [PubMed: 15842461]
- Bamias A, et al. Docetaxel and cisplatin with granulocyte colony-stimulating factor (G-CSF) versus MVAC with G-CSF in advanced urothelial carcinoma: a multicenter, randomized, phase III study from the Hellenic Cooperative Oncology Group. Journal of clinical oncology. 2004;22(2):220–228. [PubMed: 14665607]
- Bamias A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Annals.of oncology. 2013;24(4):1011–1017. [PubMed: 23136231]
- Bellmunt J, et al. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer. 1997;80(10):1966–1972. [PubMed: 9366300]
- Bellmunt J, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. Journal of Clinical Oncology. 2012;30(10):1107–1113. [PMC free article: PMC3341152] [PubMed: 22370319]
- Dogliotti L, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trial. European Urology. 2007;52(1):134–141. [PubMed: 17207911]
- Dreicer R, et al. Phase III trial of methotrexate, vinblastine, doxorubicin, and cisplatin versus carboplatin and paclitaxel in patients with advanced carcinoma of the urothelium. Cancer. 2004;100(8):1639–1645. [PubMed: 15073851]
- Galsky MD, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Annals of Oncology. 2012;23(2):406–410. [DARE provisional abstract] [PubMed: 21543626]
- Hillcoat BL, et al. A randomized trial of cisplatin versus cisplatin plus methotrexate in advanced cancer of the urothelial tract. Journal of clinical oncology. 1989;7(6):706–709. [PubMed: 2654329]
- Loehrer PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1992;10(7):1066–1073. [PubMed: 1607913]
- Lorusso V, et al. Randomised, open-label, phase II trial of paclitaxel, gemcitabine and cisplatin versus gemcitabine and cisplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium. Oncology Reports. 2005;13(2):283–287. [PubMed: 15643512]
- Mead GM, et al. A randomized trial comparing methotrexate and vinblastine (MV) with cisplatin, methotrexate and vinblastine (CMV) in advanced transitional cell carcinoma: results and a report on prognostic factors in a Medical Research Council study. MRC Advanced Bladder Cancer Working Party. British Journal of Cancer. 1998;78(8):1067–1075. [PMC free article: PMC2063167] [PubMed: 9792152]
- Petrioli R, et al. Comparison between a cisplatin-containing regimen and a carboplatin-containing regimen for recurrent or metastatic bladder cancer patients. A randomized phase II study. Cancer. 1996;77(2):344–351. [PubMed: 8625244]
- Pizzocaro G, et al. Methotrexate, vinblastine, adriamycin and cisplatin versus methotrexate and cisplatin in advanced urothelial cancer. A randomized study. European Urology. 1991;20(2):89–92. [PubMed: 1752280]
- Santis M, et al. Randomized phase II/III trial assessing gemcitabine/ carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: phase II--results of EORTC study 30986. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(33):5634–5639. [PMC free article: PMC2792956] [PubMed: 19786668]
- Santis M, et al. Randomized phase II/III trial assessing gemcitabine/ carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: phase II--results of EORTC study 30986. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(2):191–199. [PMC free article: PMC3255563] [PubMed: 22162575]
- Saxman SB, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(7):2564–2569. [PubMed: 9215826]
- Sternberg CN, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(10):2638–2646. [PubMed: 11352955]
- Sternberg CN, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. European journal of cancer (Oxford, England : 1990). 2006;42(1):50–54. [PubMed: 16330205]
- von der Maase H, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(17):3068–3077. [PubMed: 11001674]
- von der Maase H, et al. Long-term-survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. Journal of Clinical Oncology. 2005;23(21):4602–4608. (Retracted article. See vol. 16, pg. 1481, 2011) [PubMed: 16034041]
References to excluded studies (with reasons for exclusion)
- Dreicer R, et al. Vinblastine, ifosfamide, and gallium nitrate--an active new regimen in patients with advanced carcinoma of the urothelium. A phase II trial of the Eastern Cooperative Oncology Group (E5892). Cancer. 1997;79(1):110–114. Reason: not randomised trial . [PubMed: 8988734]
- McCaffrey JA, et al. Phase II randomized trial of gallium nitrate plus fluorouracil versus methotrexate, vinblastine, doxorubicin, and cisplatin in patients with advanced transitional-cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(6):2449–2455. Reason: intervention not relevant to PICO . [PubMed: 9196161]
- Andersen LJ, et al. Cisplatin, methotrexate and mitoxantrone in patients with metastatic or advanced urothelial cancer. Acta Oncologica. 1998;37(1):110–112. Reason: not randomised trial . [PubMed: 9572664]
- Mancarella S, et al. Gemcitabine/cisplatin in advanced transitional cell carcinoma of the urinary tract (TCC): A phase II multicenter trial. European Journal of Cancer. 1999;35:S347–S348. Reason: not randomised trial .
- Millikan R, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(20):4005–4013. Reason: not relevant to PICO . [PubMed: 11600601]
- Siefker-Radtke AO, et al. Phase III trial of fluorouracil, interferon alpha-2b, and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in metastatic or unresectable urothelial cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(5):1361–1367. Reason: intervention not relevant to PICO . [PubMed: 11870180]
- Stadler WM, et al. Long-term survival in phase II trials of gemcitabine plus cisplatin for advanced transitional cell cancer. Urologic Oncology. 2002;7(4):153–157. Reason: not relevant to PICO . [PubMed: 12474531]
- Amsellem-Ouazana D, et al. Management of primary resistance to gemcitabine and cisplatin (G-C) chemotherapy in metastatic bladder cancer with HER2 over-expression. Annals of Oncology. 2004;15(3):538–538. Reason: not randomised trial . [PubMed: 14998864]
- Lehmann J, et al. Adjuvant cisplatin plus methotrexate versus methotrexate, vinblastine, epirubicin, and cisplatin in locally advanced bladder cancer: results of a randomized, multicenter, phase III trial (AUO-AB 05/95). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(22):4963–4974. Reason: not relevant to PICO (adjuvant chemotherapy) [PubMed: 15939920]
- Androulakis N, et al. Sequential administration of cisplatin (C), gemcitabine (G) and docetaxel (D), as first-line treatment in patients with advanced transitional cell carcinoma (TCC) of the urothelial tract: A multicenter phase II study. Annals of Oncology. 2006;17:151–151. Reason: not randomised trial .
- Farhat FS, et al. Sequential therapy with gemcitabine and carboplatin followed by paclitaxel in first line treatment of advanced urothelial cancer. Journal of Clinical Oncology. 2008;26(15) Reason: not randomised trial .
- Apolo AB, et al. Vascular thromboembolic events in patients (pts) with advanced urothelial cancer (UC) treated with carboplatin/gemcitabine alone or in combination with bevacizumab. Journal of Clinical Oncology. 2009;27(15) Reason: not randomised trial .
- Siefker-Radtke AO. A phase II randomized four-regimen selection trial incorporating response for sequential chemotherapy in metastatic, unresectable urothelial cancer: Final results from the M. D. Anderson Cancer Center. Journal of Clinical Oncology. 2009;15 Conference(var.pagings) Reason: intervention not relevant to PICO/ abstract only insufficient data .
- Krege SR. Gemcitabine and cisplatin with or without sorafenib in urothelial carcinoma (AUO-AB 31/05). Journal of Clinical Oncology. 2010;15 Conference(var.pagings) Reason: intervention not relevant to PICO/ abstract only insufficient data .
- Brighenti M, et al. High Rate of Complete Remission (CR) Using Two Sequential, Dose-dense Regimens of Cisplatin, Gemcitabine, and Paclitaxel (CGP) Followed by HD-MVAC in Patients With Metastatic Bladder Cancer (mBC). European Journal of Cancer. 2011;47:S516–S516. Reason: not randomised trial .
- Sonpavde G. Meta-analysis of randomized trials comparing cisplatin versus carboplatin-based regimens for the first-line therapy of metastatic transitional cell carcinoma of the urothelium (TCCU). Journal of Clinical Oncology. 2011;7 Conference(var.pagings) Reason: duplicate of Gasky 2012 .
- Stadler WM, et al. Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(25):3443–3449. Reason: not relevant to PICO (adjuvant chemotherapy) [PMC free article: PMC3164246] [PubMed: 21810677]
- Yeshchina O, et al. Relative Efficacy of Perioperative Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Adriamycin, and Cisplatin in the Management of Locally Advanced Urothelial Carcinoma of the Bladder. Urology. 2012;79(2):384–390. Reason: not relevant to PICO (neoadjuvant chemotherapy) [PubMed: 22196406]
- Lin CC, et al. Efficacy of Cisplatin-Based Chemotherapy As First-Line Treatment in Asian Patients with Metastatic Urothelial Carcinoma Results of An Exploratory Subgroup Analysis of A Pool Analysis of Phase Ii/Iii Trials. Annals of Oncology. 2012;23:96–97. Reason: abstract only, insufficient information .
- Carteni G, et al. Phase II randomized trial of gemcitabine plus cisplatin (GP) and gemcitabine plus carboplatin (GC) in patients (pts) with advanced or metastatic transitional cell carcinoma of the urothelium (TCCU). Proceedings of the American Society of Clinical Oncology. 2003;384 [abstract] Reason: earlier report of Dogliotti 2007, abstract only .
- Sternberg CN, et al. Interim toxicity analysis of a randomized trial in advanced urothelial tract tumors of high-dose intensity MVAC chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC chemotherapy (EORTC 30924). Proc Annu Meet Am Soc Clin Oncol. 1997. Reason: earlier report of Sternberg 2001, abstract only . [PubMed: 11352955]
- Bellmunt J, et al. A prospective randomized trial comparing MVAC with MCAVI (methotrexate (M), carboplatin (CA), vinblastine (VI)) in patients (pts) with bladder cancer. Proceedings of the American Society of Clinical Oncology. 1993;12:237. [abstract] Abstract. Reason: earlier report of Bellmunt 2007, abstract only .
- Bellmunt J, et al. M-VAC versus M-CAVI (methotrexate (M), carboplatin (CA), vinblastine (VI)) in advanced surgically incurable bladder cancer. Proceedings of the American Society of Clinical Oncology. 1996;15:265. [abstract] Abstract. Reason: earlier report of Bellmunt 2007, abstract only .
- Scher HI. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. The Journal of urology. 1992;148(5):1625–1626. Reason: editorial comment on Loehrer (1992) [PubMed: 1433578]
- von der Maase H, et al. Health care resource use for patients with advanced bladder cancer treated with gemcitabine plus cisplatin (GC) versus MVAC in a phase III trial. Annals of Oncology. 2000;11:74–74. Reason: duplicate data of von der Maase (2000) abstract only .
- Logothetis CJ, et al. Escalated MVAC with or without recombinant human granulocyte-macrophage colony-stimulating factor for the initial treatment of advanced malignant urothelial tumors: results of a randomized trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1995;13(9):2272–2277. Reason: Outcome not relevant to PICO (dose intensity) [PubMed: 7666085]
- Bamias A, et al. Docetaxel and cisplatin versus M-VAC in advanced urothelial carcinoma: a multicenter, randomized, phase III study conducted by the Hellenic Cooperative Oncology Group. Proceedings of the American Society of Clinical Oncology. 2003;384 [abstract] Reason: earlier report of Bamias (2004) abstract only .
- Boyer MJ, et al. Randomized phase II study of gemcitabine (G) with either carboplatin (C) or docetaxel (D) in patients with locally advanced or metastatic transitional cell carcinoma (TCC) of the urothelium: preliminary results. Proceedings of the American Society of Clinical Oncology. 2003;390 [abstract] Reason: immature data, abstract only .
- Bamias A. Biweekly carboplatin/gemcitabine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: Report of efficacy, quality of life and geriatric assessment. Oncology. 2007;73(5-6):290–297. Reason: not RCT . [PubMed: 18477854]
- Dodd PM, et al. Evaluation of drug delivery and survival impact of dose-intense relative to conventional-dose methotrexate, vinblastine, doxorubicin, and cisplatin chemotherapy in urothelial cancer. Cancer Investigation. 2000;18(7):626–634. Reason: not RCT . [PubMed: 11036470]
- Culine S, et al. Reducing the time interval between cycles using standard doses of docetaxel and lenogastrim support: a feasibility study. Cancer. 2004;101(1):178–182. Reason: feasibility study, majority breast cancer patients . [PubMed: 15222004]
- Oudard S, et al. Multicenter Randomized Phase 2 Trial of Gemcitabine - Platinum with Or Without Trastuzumab (T) in Advanced/Metastatic Urothelial Carcinoma (A/Muc) with Her2 Overexpression. Annals of Oncology. 2012;23:259–259. Reason: intervention not relevant to PICO (Trastuzumab)
- Roychowdhury DF, Hayden A, Liepa AM. Health-related quality-of-life parameters as independent prognostic factors in advanced or metastatic bladder cancer. Journal of Clinical Oncology. 2003;21(4):673–678. Reason: QoL data from van der Maase – prognostic factor analysis, no extra data reported . [PubMed: 12586805]
- Lehmann J. Is there standard chemotherapy for metastatic bladder cancer? Quality of life and medical resources utilization based on largest to date randomized trial. Critical Reviews in Oncology/Hematology. 2003;47(2):171–179. Reason: QoL data from van der Maase – no extra data reported . [PubMed: 12900010]
- Gagliano R, et al. Adriamycin versus adriamycin plus cis-diamminedichloroplatinum (DDP) in advanced transitional cell bladder carcinoma. A Southwest Oncology Group study. American Journal of Clinical Oncology. 1983;6(2):215–218. Reason: Intervention not relevant to PICO (DDP) [PubMed: 6681934]
- Soloway MS, et al. A comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A Study. Cancer. 1983;52(5):767–772. Reason: Intervention not relevant to PICO (cyclophosphamide) [PubMed: 6347356]
- Troner M, et al. Phase III comparison of cisplatin alone versus cisplatin, doxorubicin and cyclophosphamide in the treatment of bladder (urothelial) cancer: a Southeastern Cancer Study Group trial. The Journal of urology. 1987;137(4):660–662. Reason: Intervention not relevant to PICO (cyclophosphamide) [PubMed: 3550148]
- Logothetis CJ, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1990;8(6):1050–1055. Reason: Intervention not relevant to PICO (CISCA) [PubMed: 2189954]
- Wit R, et al. Randomised phase II trial of carboplatin and iproplatin in advanced urothelial cancer. European journal of cancer (Oxford, England : 1990). 1991;27(11):1383–1385. Reason: Intervention not relevant to PICO (Iproplatin) [PubMed: 1835851]
- Kuroda M, et al. Efficacy of dose-intensified MEC (methotrexate, epirubicin and cisplatin) chemotherapy for advanced urothelial carcinoma: a prospective randomized trial comparing MEC and M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin). Japanese Urothelial Cancer Research Group. Japanese Journal of Clinical Oncology. 1998;28(8):497–501. Reason: Intervention not relevant to PICO (epirubicin) [PubMed: 9769784]
- Culine S, et al. Gemcitabine or gemcitabine plus oxaliplatin in the first-line treatment of patients with advanced transitional cell carcinoma of the urothelium unfit for cisplatin-based chemotherapy: a randomized phase 2 study of the French Genitourinary Tumor Group (GETUG V01). European Urology. 2011;60(6):1251–1257. Reason: Intervention not relevant to PICO (oxaliplatin) [PubMed: 21924547]
- von der Maase H, et al. Long-term-survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. Annals of Oncology. 2011;22(11):2536–2536. (Retraction of vol 23, pg 4602, 2005) Reason: retraction of Roberts (2006), not a study reference . [PubMed: 16034041]
- Kwak H. The comparison of the efficacy and side effects between M-VAC and GC chemotherapy for advanced or metastatic urothelial carcinoma patients with a good performance status. Korean Journal of Urology. 2007;48(12):1229–1235. Reason: foreign language, not RCT .
- BA11: Randomised phase III study comparing paclitaxel/cisplatin/gemcitabine and cisplatin/gemcitabine in patients with metastatic or locally advanced urothelial cancer without prior systemic therapy. UroOncology. 2002;2(2):55–56. Reason: study record, no data .
- von der MH, et al. Do elderly patients with advanced urothelial carcinoma benefit from platinum-based chemotherapy? Nature Clinical Practice Urology. 2005;2(7):318–319. Reason: comment on Bamias (2005)
- Bamias A, et al. The outcome of elderly patients with advanced urothelial carcinoma after platinum-based combination chemotherapy. Annals of Oncology. 2005;16(2):307–313. Reason: not randomised trial . [PubMed: 15668289]
Evidence tables
BCa, bladder cancer; TCC, transitional cell cancer; GCarbo, Gemcitabine/Carboplatin; M-CAVI, Methotrexate/Carboplatin/Vinblastine; PS, performance status; GFR, Glomerular filtration rate; GC, Gemcitabine/Cisplatin; GCP, Gemcitabine/Cisplatin/Paclitaxel; MVAC, Methotrexate/Vinblastine/Adriamycin/Cisplatin; CaP, Carboplatin/Paclitaxel; HD-MVAC, High-dose
Methotrexate/Vinblastine/Adriamycin/Cisplatin; NCI-CTC, National Cancer Institute Common Toxicity Criteria; GCSF, granulocyte colony-stimulating factor; CISCA, Cisplatin/Doxorubicin/Cyclophosphamide; SAT, severe acute toxicity
Download PDF (367K)
Health Economic Evidence: What are the comparative patient outcomes for treating metastatic bladder cancer with first-line chemotherapy
Review questions
What is the optimal first-line chemotherapy regimen for patients with incurable locally advanced or metastatic bladder cancer?
Table 136Pico Table For The Optimal First-Line Chemotherapy Regimens For Treating Metastatic Bladder Cancer
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with incurable locally advanced or metastatic bladder cancer Cisplatin fit (GFR >60 PS 0/1) | Chemotherapy agents for first-line chemotherapy (alone or in combination):
| Each other (Cisplatin vrs Non Cisplatin) No treatment |
Information sources and eligibility criteria
The following databases were searched for economic evidence relevant to the PICO: MEDLINE, EMBASE, COCHRANE, NHS EED and HEED. Studies conducted in OECD countries other than the UK were considered.
Studies were selected for inclusion in the evidence review if the following criteria were met:
- Both cost and health consequences of interventions reported (i.e. true cost-effectiveness analyses)
- Conducted in an OECD country
- Incremental results are reported or enough information is presented to allow incremental results to be derived
- Studies that matched the population, interventions, comparators and outcomes specified in PICO
- Studies that meet the applicability and quality criteria set out by NICE, including relevance to the NICE reference case and UK NHS
Note that studies that measured effectiveness using quality of life based outcomes (e.g. QALYs) were desirable but, where this evidence was unavailable, studies using alternative effectiveness measures (e.g. life years) were considered.
Selection of studies
The literature search results were screened by checking the article's title and abstract for relevance to the review question. The full articles of non-excluded studies were then attained for appraisal and compared against the inclusion criteria specified above.
Results
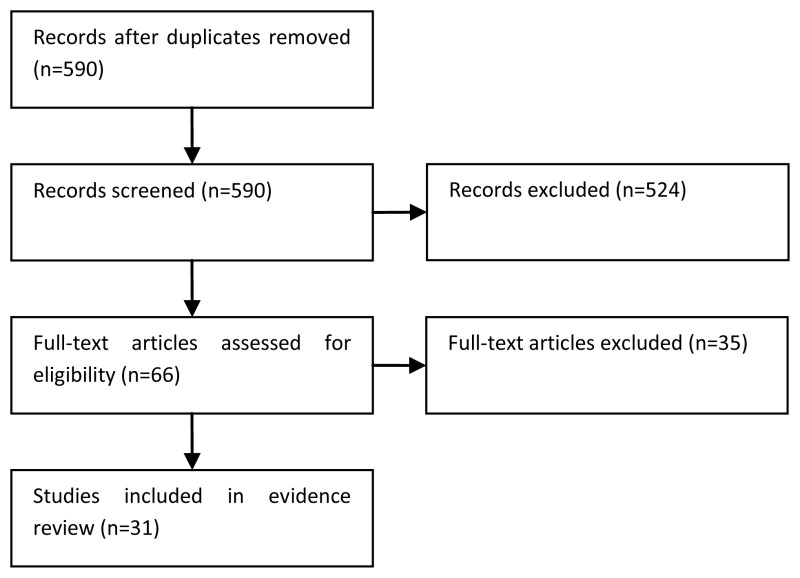
Three searches for economic evidence were run over the development of the guideline; one at the start of the process, an update midway through and a further update at the end of the process. The diagram below shows the combined results of the three searches and illustrates the sifting process.
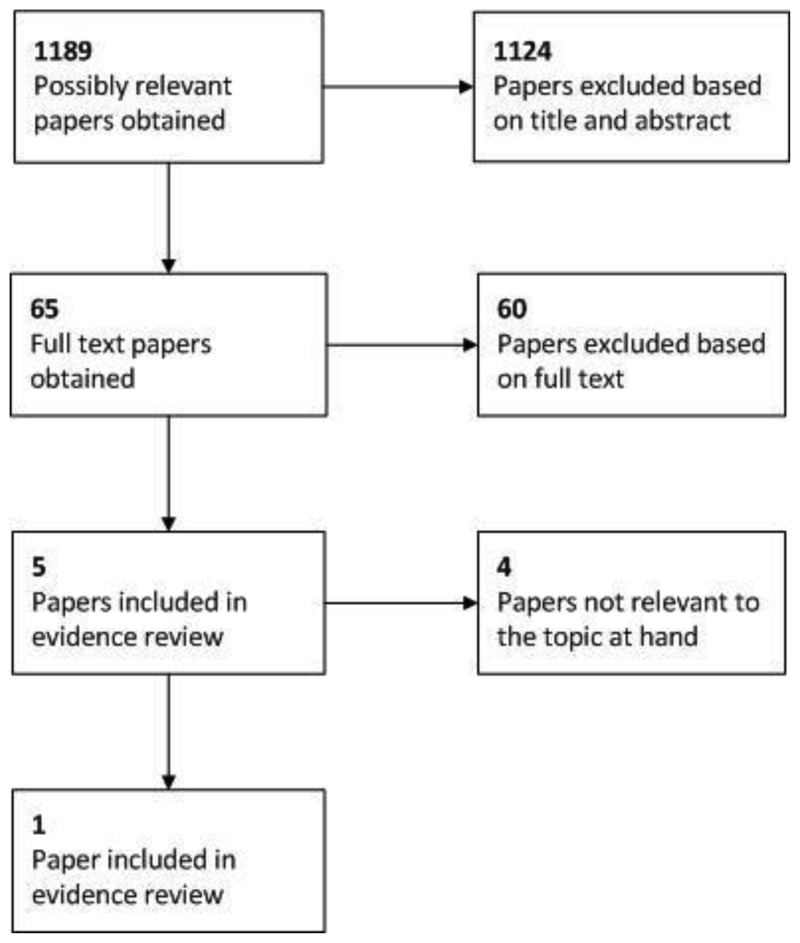
It can be seen that, in total, 1,189 possibly relevant papers were identified. Of these, 1,124 papers were excluded at the initial sifting stage based on the title and abstract while 65 full papers were obtained for appraisal. A further 56 papers were excluded based on the full text as they were not applicable to the PICO or did not include an incremental analysis of both costs and health effects. Therefore, nine papers were included in the systematic review of the economic evidence for this guideline.
One of these nine papers related to the topic at hand and was thus included in the review of published economic evidence for this topic; Robinson et al. 2004. The study included a cost-effectiveness analysis where effectiveness was measured using quality adjusted life years (QALYs) i.e. a cost-utility analysis.
Quality and applicability of the included study
Robinson et al. 2004 was deemed to be only partially applicable to the decision problem that we are evaluating because the utility values were not directly reported by patients (as recommended by NICE). Instead they were elicited from healthcare professionals.
Potentially serious limitations were identified with the analysis. In particular, a potential conflict of interest was identified as the study was funded by the manufacturer of one of the therapies under consideration (Eli Lilly and Co, manufacturers of Gemcitabine). In addition, further sensitivity analysis could have been conducted to better explore uncertainty.
Table 137Table Showing Methodological Quality And Applicability Of The Included Study
| Methodological quality | Applicability | |
|---|---|---|
| Directly applicable | Partially applicable | |
| Minor limitations | ||
| Potentially serious limitations | Robinson et al. 2004 | |
| Very serious limitations | ||
Modified GRADE table
The primary results of the analysis by Robinson et al. 2004 are summarised in the modified GRADE table below.
Table 138
Modified Grade Table Showing The Included Evidence On The Optimal First-Line Chemotherapy Regimens For Treating Metastatic Bladder Cancer.
Evidence statements
The base case results of the cost-effectiveness analysis showed that, in comparison to the MVAC regimen, the combination of gemcitabine and cisplatin provided one additional quality adjusted life year (QALY) at a cost of £22,925. This ICER value is slightly higher than the threshold typically adopted by NICE (£20,000 per QALY) and so gemcitabine and cisplatin would not strictly be considered cost-effective.
Exceptions are made in instances where there may be some aspects that are not captured in the model. In this case, the cost of gemcitabine used in the model is unlikely to reflect the cost in current practice as the drug has come off patent in the intervening years. With the lower cost of gemcitabine in current practice, it is possible that the cost-effectiveness result would be improved significantly and could fall below the threshold of £20,000 per QALY.
However, there were concerns about the utility values that were used in the model as they were derived from healthcare professionals rather than patients and thus the QALY estimates may be unreliable. Furthermore, the applicability of this study to current practice is debatable as the MVAC regimen used in the study has largely been replaced with a more efficacious accelerated MVAC regimen.
Thus, overall, the available evidence base was not considered to provide a reliable estimate of cost-effectiveness that is relevant to current clinical practice.
References
- 1.
- Robinson P, Maase Hv, Bhalla S, Kielhorn A, Artistides M, Brown A, Tilden D. Cost-utility analysis of the GC versus MVAC regimens for the treatment of locally advanced or metastatic prostate cancer. Expert Rev Pharmacoecon Outcomes Res. 2004;4(1):27–38. [PubMed: 19807333]
Full evidence table
The full details of the study included in the evidence review are presented in the evidence table below.
Table 139
Full evidence table showing the included evidence on the optimal first-line chemotherapy regimens for treating metastatic bladder cancer.
5.1.2. Post-first line chemotherapy
Review question: What is the optimal post first-line chemotherapy regimen for patients with incurable locally advanced or metastatic bladder cancer?
Rationale
First line chemotherapy for metastatic disease is widely accepted as appropriate treatment for at least a proportion of patients.
Management of patients who progress on or relapse after 1st line treatment is much more controversial. Prognosis is poor with median survivals measured in a few months. There is a wide variety of practice in whether to offer 2nd line therapy to such patients. It is likely response rates are less; and toxicity may be higher thus questioning the clinical benefits of treatment. A key question is first therefore whether there is a role for further chemotherapy in some or all patients? If so can we identify the patients that are most likely to benefit and/or those in which chemotherapy is ineffective and treatment be avoided.
If patients are thought suitable for chemotherapy what form should this be? Should patients be re-challenged with initial chemotherapy or alternative combination regime (eg MVAC if Gemcitabine/cisplatin) was used first. Are other alternatives likely to be as effective (eg Paclitaxel) even though not licensed? Are single drugs better or worse option than combination?
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with incurable locally advanced or metastatic bladder cancer that has progressed following first line chemotherapy | Chemotherapy agents for second-line chemotherapy (alone or in combination): Paclitaxel, Irinotecan, Bortezomib, Pemetrexed, Oxaliplatin, Ifosfamide, Lapatinib, Docetaxel, Gemcitabine, Topotecan, Carboplatin, Gefitinib, Sorafenib, Sunitinib, MVAC (vinflunine for search) | Each other best supportive care |
METHODS
Information sources
A literature search was performed by the information specialist (EH).
Selection of studies
The information specialist (EH) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. The full articles were then obtained for potentially relevant studies and checked against the inclusion criteria. Randomised trials and single-arm phase II studies were selected for this review question.
Data synthesis
Data from comparative studies were extracted into RevMan and risk ratios were calculated where possible.
RESULTS
Study quality and results
The included evidence is summarised in Tables 140-170.
Table 140
GRADE evidence profile: Topotecan for second-line chemotherapy.
Table 141
GRADE evidence profile: Iritonecan for second-line chemotherapy.
Table 142
GRADE evidence profile: Lapatanib for second-line chemotherapy.
Table 143
GRADE evidence profile: Bortezomib for second-line chemotherapy.
Table 144
GRADE evidence profile: Sorafenib for second-line chemotherapy.
Table 145
GRADE evidence profile: Oxaliplatin for second-line chemotherapy.
Table 146
GRADE evidence profile: Pemetrexed for second-line chemotherapy.
Table 147
GRADE evidence profile: Docetaxel for second-line chemotherapy.
Table 148
GRADE evidence profile: Ifosfamide for second-line chemotherapy.
Table 149
GRADE evidence profile: Sunitinib for second-line chemotherapy.
Table 150
GRADE evidence profile: Paclitaxel for second-line chemotherapy.
Table 151
GRADE evidence profile: Gemcitabine for second-line chemotherapy.
Table 152
GRADE evidence profile: Gemcitabine & Paclitaxel for second-line chemotherapy.
Table 153
GRADE evidence profile: Short-term versus prolonged gemcitabine and paclitaxel.
Table 154
GRADE evidence profile: Paclitaxel & Carboplatin for second-line chemotherapy.
Table 155
GRADE evidence profile: Methotrexate, vinblastine, doxorubicin, cisplatin (MVAC) for second-line chemotherapy.
Table 156
GRADE evidence profile: Gemcitabine, cisplatin for second-line chemotherapy.
Table 157
GRADE evidence profile: Paclitaxel, cisplatin, methotrexate for second-line chemotherapy.
Table 158
GRADE evidence profile: Paclitaxel, cisplatin for second-line chemotherapy.
Table 159
GRADE evidence profile: Methotrexate, paclitaxel for second-line chemotherapy.
Table 160
GRADE evidence profile: Paclitaxel, ifosfamide for second-line chemotherapy.
Table 161
GRADE evidence profile: Docetaxel, ifosfamide for second-line chemotherapy.
Table 162
GRADE evidence profile: Docetaxel, oxaliplatin for second-line chemotherapy.
Table 163
GRADE evidence profile: Cisplatin, Gemcitabine & Ifosfamide for second-line chemotherapy.
Table 164
GRADE evidence profile: Gemcitabine, Ifosfamide for second-line chemotherapy.
Table 165
GRADE evidence profile: Gemcitabine, Docetaxel for second-line chemotherapy.
Table 166
GRADE evidence profile: Gemcitabine, carboplatin, docetaxel for second-line chemotherapy.
Table 167
GRADE evidence profile: Methotrexate, Paclitaxel, Epirubicin, Carboplatin for second-line chemotherapy.
Table 168
GRADE evidence profile: Best supportive care after progression from first-line chemotherapy.
Table 169
Single-agent second-line chemotherapy trials in advanced bladder cancer.
Table 170
Multi-agent second-line trials in advanced bladder cancer.
Evidence statements
Single-agent chemotherapy
Very low quality evidence for Topotecan, Iritonecan, Lapatanib, Sorefanib, Oxaliplatin and Sunitinib was provided by one non-comparative phase II study for each regimen. Overall survival ranged from 4.2 months (Lapatanib) to 7.1 months (Sunitinib). Progression-free survival ranged from 1.5 months (Topotecan) to 2.4 months (Sunitinib). Overall tumour response rate was highest for Topotecan at 9%. Toxicity rates were highest for Topotecan with 43%, 61%, and 77% of participants developing grade 3-4 thrombocytopenia, anaemia, and leucopenia, respectively. Two studies (46 participants) provided very low quality evidence on Bortezomib, with median overall survival durations of 3.5 months (Gomez-Aubin et al., 2007) and 5.7 months (Rosenberg et al., 2008). Both studies were closed early due to a lack of tumour response to the treatment, with no responses reported in either study. One study (47 participants) provided very low quality evidence of Pemetrexed, with a median overall survival of 9.2 months and a response rate of 28% for those previously treated in the metastatic setting (Sweeny et al., 2006). A second smaller study (13 participants) of Pemetrexed reported a lower response rate of 8% (Galsky et al., 2007). Across both studies, 12% of participants reported grade 3-4 neutropenia and thrombocytopenia. Very low quality evidence for Gemcitabine was provided by four studies (133 participants), with overall survival ranging from 5 months to 13 months across studies and an overall tumour response of 22%. Grade 3-4 neutropenia was the most common adverse event (37% of participants) (2 studies, 79 participants). In one study (Albers et al., 2002), 25 participants reported health-related quality of life, where responders to Gemcitabine showed an improvement in pain score from 4.3 to 5.8 on a 7-point scale. In contrast, non-responders reported an increase in pain during treatment.
Multi-agent chemotherapy
The combination of Gemcitabine and Paclitaxel (GP) was reported by 6 studies (109 participants, very low quality evidence). The overall response rate was 30%, with median overall survival ranging from 8 months to 12.4 months. One study reported a median progression-free survival of 6.1 months (Ikeda et al., 2011). Four studies reported grade 3-4 neutropenia, with an overall rate of 42%. One randomised phase III trial (Albers et al., 2011) and one randomised phase II trial (Fechner et al., 2006) provided low quality evidence of short-term (three-week schedule) versus prolonged (maintenance until progression) GP regimes (123 participants). No differences in overall survival and progression-free survival were reported between trial arms. In the phase III trial median overall survival was 7.8 months in the subgroup of patients who had first-line chemotherapy for metastatic cancer (Albers et al., 2011). The pooled overall tumour response rate was 41% in both trial arms. Grade 3-4 leucopenia was the most common toxicity with no difference in rate between short-term and maintenance GP treatment (36% versus 23%). Two treatment-related deaths were reported on the prolonged GP arm in the phase III study. Several small non-randomised studies providing very low quality evidence, generally show that other non-platinum based regimens (e.g. Methotrexate & Paclitaxel; Paclitaxel & Ifosfamide; Docetaxel & Ifosfamide; Docetaxel & Oxaliplatin; Gemcitabine & Ifosfamide; Gemcitabine & Docetaxel) have lower response rates and overall survival durations than Gemcitabine and Paclitaxel.
Three studies (93 participants) reported very low quality evidence about Carboplatin and Paclitaxel, with median overall survival ranging from six to 11 months, and an overall response rate of 25%. Progression-free survival was around four months in all three studies. Grade 3-4 neutropenia was reported in 50 out of 93 (54%) participants. Health-related quality of life was reported by one study, where there were no differences between pre-treatment and post-treatment scores on the EORTC-QLQ C30. Cisplatin based multi-agent chemotherapy regimens (MVAC; Gemcitabine & Cisplatin (GC); Paclitaxel, Methotrexate & Cisplatin (PMC); Paclitaxel & Cisplatin; Cisplatin, Gemcitabine & Ifosfamide) produced response rates of 30% to 40% and overall survival durations of 9.5 to 11 months (very low quality evidence). Rates of grade 3-4 neutropenia were 30%-67% and rates of grade 3-4 thrombocytopenia were 30%-32% for MVAC, GC and PMC. Lower toxicity rates were reported for the regimen of Paclitaxel & Cisplatin, with 5% grade 3-4 neutropenia and 1% grade 3-4 thrombocytopenia and anaemia (Uhm et al., 2007). One study (26 participants, very low quality evidence) reported a median overall survival and progression-free survival of 12.6 months and 5 months with Gemcitabine, Carboplatin & Docetaxel (Tsuruta et al., 2011). Excluding those who had received combination radiation therapy, the overall tumour response rate was 56%. Toxicity data was not reported separately for patients receiving second-line chemotherapy. Grade 3-4 neutropenia was reported in 80% of participants, thrombocytopenia in 51%, and anaemia in 43%. There were no treatment-related deaths.
Best supportive care
Moderate quality evidence from the control arm of a phase III randomised trial reported a median overall survival of 4.6 months and a median progression-free survival of 1.5 months for 117 participants receiving best supportive care for progression after first-line chemotherapy (Bellmunt et al., 2009). There were no tumour responses. One patient reported grade 3-4 neutropenia and one patient reported grade 3-4 thrombocytopenia. Nine participants reported grade 3-4 anaemia. Health-related quality of life as measured by the EORTC QLQ-C30, decreased continuously from baseline through to week 18 (mean scores were not reported).
References to included studies
- Akaza H. Efficacy and safety of gemcitabine monotherapy in patients with transitional cell carcinoma after cisplatin-containing therapy: A Japanese experience. Japanese Journal of Clinical Oncology. 2007;37(3):201–206. [PubMed: 17452426]
- Albers P, et al. Gemcitabine monotherapy as second-line treatment in cisplatin-refractory transitional cell carcinoma - prognostic factors for response and improvement of quality of life. Onkologie. 2002;25(1):47–52. [PubMed: 11893883]
- Albers P, et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99] Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22(2):288–294. [PubMed: 20682548]
- Beer TM. Southwest Oncology Group phase II study of irinotecan in patients with advanced transitional cell carcinoma of the urothelium that progressed after platinum-based chemotherapy. Clinical Genitourinary Cancer. 2008;6(1):36–39. [PubMed: 18501081]
- Bellmunt J, et al. Feasibility trial of methotrexate-paclitaxel as a second line therapy in advanced urothelial cancer. Cancer Investigation. 2002;20(5-6):673–685. [PubMed: 12197223]
- Bellmunt J, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(27):4454–4461. [PubMed: 19687335]
- Sweeney CJ, Roth BJ, Kabbinavar FF, Vaughn DJ, Arning M, Curiel RE, Obasaju CK, Wang Y, Nicol SJ, Kaufman DS. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J.Clin.Oncol. 2006;24(21):3451–3457. [PubMed: 16849761]
- Choueiri TK, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. Journal of Clinical Oncology. 2012;30(5):507–512. [PMC free article: PMC4104290] [PubMed: 22184381]
- Gallagher DJ. Phase II study of sunitinib in patients with metastatic urothelial cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(8):1373–1379. [PubMed: 20142593]
- Dreicer R, et al. Phase II trial of gemcitabine and docetaxel in patients with advanced carcinoma of the urothelium: a trial of the Eastern Cooperative Oncology Group. Cancer. 2003;97(11):2743–2747. [PubMed: 12767086]
- Dreicer R. Phase 2 trial of sorafenib in patients with advanced urothelial cancer: A Trial of the Eastern Cooperative Oncology Group. Cancer. 2009;115(18):4090–4095. [PMC free article: PMC2774800] [PubMed: 19536901]
- Fechner G, et al. Randomised phase II trial of gemcitabine and paclitaxel second-line chemotherapy in patients with transitional cell carcinoma (AUO Trial AB 20/99). International Journal of Clinical Practice. 2006;60(1):27–31. [PubMed: 16409425]
- Gomez-Abuin G, Winquist E, Stadler WM, Pond G, Degendorfer P, Wright J, Moore MJ. A phase II study of PS-341 (Bortezomib) in advanced or metastatic urothelial cancer A trial of the Princess Margaret Hospital and University of Chicago phase II consortia. Invest.New Drugs. 2007;25185(2):181. [PubMed: 16983508]
- Galsky MD. Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Investigational New Drugs. 2007;25(3):265–270. [PubMed: 17146733]
- Gebbia V, et al. Single agent 2',2'-difluorodeoxycytidine in the treatment of metastatic urothelial carcinoma: a phase II study. La Clinica terapeutica. 1999;150(1):11–15. [PubMed: 10367539]
- Gondo T, et al. The efficacy and safety of gemcitabine plus cisplatin regimen for patients with advanced urothelial carcinoma after failure of M-VAC regimen. International Journal of Clinical Oncology. 2011;16(4):345–351. [PubMed: 21331770]
- Halim A. Methotrexate-paclitaxel-epirubicin-carboplatin as second-line chemotherapy in patients with metastatic transitional cell carcinoma of the bladder pretreated with cisplatin-gemcitabine: A phase II study. Asia-Pacific Journal of Clinical Oncology. 2013;9(1):60–65. [PubMed: 22897883]
- Han KS, et al. Methotrexate, vinblastine, doxorubicin and cisplatin combination regimen as salvage chemotherapy for patients with advanced or metastatic transitional cell carcinoma after failure of gemcitabine and cisplatin chemotherapy. British Journal of Cancer. 2008;98(1):86–90. [PMC free article: PMC2359702] [PubMed: 18087289]
- Ikeda M, et al. Combination of gemcitabine and paclitaxel is a favorable option for patients with advanced or metastatic urothelial carcinoma previously treated with cisplatin-based chemotherapy. Japanese Journal of Clinical Oncology. 2011;41(10):1214–1220. [PubMed: 21903707]
- Joly F, et al. Do patients with advanced urothelial carcinoma benefit from weekly paclitaxel chemotherapy? A GETUG phase II study. Clinical Genitourinary Cancer. 2009;7(2):E28–E33. [PubMed: 19692319]
- Kanai K, et al. Gemcitabine and paclitaxel chemotherapy for advanced urothelial carcinoma in patients who have received prior cisplatin-based chemotherapy. International Journal of Clinical Oncology. 2008;13(6):510–514. [PubMed: 19093178]
- Kaufman DS, et al. A multi-institutional phase II trial of gemcitabine plus paclitaxel in patients with locally advanced or metastatic urothelial cancer. Urologic Oncology. 2004;22(5):393–397. [PubMed: 15464919]
- Kouno T. Weekly Paclitaxel and Carboplatin against Advanced Transitional Cell Cancer after Failure of a Platinum-Based Regimen. European Urology. 2007;52(4):1115–1122. [PubMed: 17433855]
- Krege S, et al. Docetaxel and ifosfamide as second line treatment for patients with advanced or metastatic urothelial cancer after failure of platinum chemotherapy: a phase 2 study. Journal of Urology. 2001;165(1):67–71. [PubMed: 11125366]
- Lin CC. Gemcitabine and ifosfamide as a second-line treatment for cisplatin-refractory metastatic urothelial carcinoma: A phase II study. Anti-Cancer Drugs. 2007;18(4):487–491. [PubMed: 17351402]
- Lorusso V, et al. A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Italian Co-operative Group on Bladder Cancer. European Journal of Cancer. 1998;34(8):1208–1212. [PubMed: 9849481]
- McCaffrey JA, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. Journal of Clinical Oncology. 1997;15(5):1853–1857. [PubMed: 9164195]
- Pronzato P, Vigani A, Pensa F, Vanoli M, Tani F, Vaira F. Second line chemotherapy with ifosfamide as outpatient treatment for advanced bladder cancer. Am.J.Clin.Oncol. 1997;20(5):519–521. [PubMed: 9345341]
- Pagliaro LC, et al. Cisplatin, gemcitabine, and ifosfamide as weekly therapy: a feasibility and phase II study of salvage treatment for advanced transitional-cell carcinoma. Journal of Clinical Oncology. 2002;20(13):2965–2970. [PubMed: 12089226]
- Papamichael D, et al. Phase II study of paclitaxel in pretreated patients with locally advanced/metastatic cancer of the bladder and ureter. British Journal of Cancer. 1997;75(4):606–607. [PMC free article: PMC2063294] [PubMed: 9052419]
- Pectasides D, et al. Combination chemotherapy with gemcitabine and ifosfamide as second-line treatment in metastatic urothelial cancer. A phase II trial conducted by the Hellenic Cooperative Oncology Group. Annals of Oncology. 2001;12(10):1417–1422. [PubMed: 11762814]
- Witte RS, Manola J, Burch PA, Kuzel T, Weinshel EL, Loehrer PJ Sr. Topotecan in previously treated advanced urothelial carcinoma: an ECOG phase II trial. Invest.New Drugs. 1998;16(2):191–195. [PubMed: 9848585]
- Rosenberg JE. Phase II study of bortezomib in patients with previously treated advanced urothelial tract transitional cell carcinoma: CALGB 90207. Annals of Oncology. 2008;19(5):946–950. [PMC free article: PMC5815560] [PubMed: 18272914]
- Soga N, et al. Paclitaxel Carboplatin chemotherapy as a second-line chemotherapy for advanced platinum resistant urothelial cancer in Japanese cases. International Journal of Urology. 2007;14(9):828–832. [PubMed: 17760750]
- Srinivas SH. A phase II study of docetaxel and oxaliplatin for second-line treatment of urothelial carcinoma. Chemotherapy. 2009;55(5):321–326. [PMC free article: PMC2814022] [PubMed: 19641314]
- Sternberg CN, et al. Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer. 2001;92(12):2993–2998. [PubMed: 11753976]
- Suyama T, et al. Combination of gemcitabine and paclitaxel as second-line chemotherapy for advanced urothelial carcinoma. Japanese Journal of Clinical Oncology. 2009;39(4):244–250. [PubMed: 19211575]
- Sweeney CJ, et al. A Phase II study of paclitaxel and ifosfamide for patients with advanced refractory carcinoma of the urothelium. Cancer. 1999;86(3):514–518. [PubMed: 10430261]
- Takahashi T, et al. Biweekly paclitaxel and gemcitabine for patients with advanced urothelial cancer ineligible for cisplatin-based regimen. Japanese Journal of Clinical Oncology. 2006;36(2):104–108. [PubMed: 16418182]
- Tsuruta H, et al. Combination therapy consisting of gemcitabine, carboplatin, and docetaxel as an active treatment for advanced urothelial carcinoma. International Journal of Clinical Oncology. 2011;16(5):533–538. [PubMed: 21431341]
- Tu SM, et al. Paclitaxel, cisplatin and methotrexate combination chemotherapy is active in the treatment of refractory urothelial malignancies. Journal of Urology. 1995;154(5):1719–1722. [PubMed: 7563331]
- Uhm JE, et al. Paclitaxel with cisplatin as salvage treatment for patients with previously treated advanced transitional cell carcinoma of the urothelial tract. Neoplasia. 2007;9(1):18–22. [PMC free article: PMC1804323] [PubMed: 17325740]
- Vaishampayan UN. Phase II trial of carboplatin and paclitaxel in cisplatin-pretreated advanced transitional cell carcinoma: A Southwest Oncology Group study. Cancer. 2005;104(8):1627–1632. [PubMed: 16138364]
- Vaughn DJ, et al. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. Journal of Clinical Oncology. 2002;20(4):937–940. [PubMed: 11844814]
- Winquist EV. A Phase II study of oxaliplatin in urothelial cancer. Urologic Oncology: Seminars and Original Investigations. 2005;23(3):150–154. [PubMed: 15907713]
- Witte RS, PJ Eastern cooperative oncology group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinoma. Journal of Clinical Oncology. 1997;15(2):589–593. [PubMed: 9053481]
- Wulfing C. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115(13):2881–2890. [PubMed: 19399906]
References to excluded studies (with reasons for exclusion)
- Gorelov A, et al. Paclitaxel injectable emulsion: Phase 2a study of weekly administration in patients with metastatic or locally advanced unresectable or recurrent urothelial transitional cell cancer (TCC). Journal of Clinical Oncology. 2004;22(14):403S–403S. Reason: immature data, abstract only .
- Albers P, et al. Randomized phase II trial of gemcitabine and paclitaxel with or without maintenance treatment in patients with cisplatin refractory transitional cell carcinoma. Proceedings of the American Society of Clinical Oncology. 2002;21(Pt 1):200a. [abstract] Abstract. Reason: abstract only, superseded by randomised phase III trial .
- Dreicer R, et al. Paclitaxel in advanced urothelial carcinoma: its role in patients with renal insufficiency and as salvage therapy. Journal of Urology. 1996;156(5):1606–1608. Reason: 3/9 patients for 2nd line chemo (results not reported separately) [PubMed: 8863548]
- Edeline J, et al. Accelerated MVAC chemotherapy in patients with advanced bladder cancer previously treated with a platinum-gemcitabine regimen. European Journal of Cancer. 2012;48(8):1141–1146. Reason: retrospective study . [PubMed: 22364733]
- Matsumoto K, et al. Gemcitabine and paclitaxel chemotherapy as a second-line treatment for advanced or metastatic urothelial carcinoma. International Journal of Urology. 2007;14(11):1000–1004. Reason: early report of Ikeda (2011) [PubMed: 17956525]
- Culine S, et al. Combining Paclitaxel and Lapatinib as Second-line Treatment for Patients with Metastatic Transitional Cell Carcinoma: A Case Series. Anticancer Research. 2012;32(9):3949–3952. Reason: 6 retrospective case studies . [PubMed: 22993342]
- Einhorn LH, Roth BJ, Ansari R, Dreicer R, Gonin R, Loehrer PJ. Phase II trial of vinblastine, ifosfamide, and gallium combination chemotherapy in metastatic urothelial carcinoma. J Clin Oncol. 1994;122276(11):2271. Reason: intervention not relevant to PICO (Gallium) [PubMed: 7525884]
- Joung JY, et al. Paclitaxel and cisplatin chemotherapy for metastatic urothelial carcinoma after failure of two courses of platinum-based regimens. International Journal of Urology. 2011;18(5):350–357. Reason: retrospective study (3rd line chemo) [PubMed: 21355894]
- Meluch AA, et al. Paclitaxel and gemcitabine chemotherapy for advanced transitional-cell carcinoma of the urothelial tract: a phase II trial of the Minnie pearl cancer research network. Journal of Clinical Oncology. 2001;19(12):3018–3024. Reason: 15/54 patients recieved 2nd line chemo – survival and toxicity data not reported separately . [PubMed: 11408496]
- Miyata Y, et al. Use of low-dose combined therapy with gemcitabine and paclitaxel for advanced urothelial cancer patients with resistance to cisplatin-containing therapy: a retrospective analysis. Cancer Chemotherapy and Pharmacology. 2012;70(3):451–459. Reason: retrospective study . [PMC free article: PMC3428519] [PubMed: 22864875]
- Otto T, et al. Paclitaxel-based second-line therapy for patients with advanced chemotherapy-resistant bladder carcinoma (M1): a clinical Phase II study. Cancer. 1997;80(3):465–470. Reason: intervention not relevant to PICO (acellular pertussis vaccine) [PubMed: 9241080]
- Saito K, et al. Impact of C-reactive protein kinetics on survival of patients with advanced urothelial carcinoma treated by second-line chemotherapy with gemcitabine, etoposide and cisplatin. BJU International. 2012;110(10):1478–1484. Reason: intervention not relevant to PICO (etoposide) [PubMed: 22520732]
- Srinivas S, Guardino AE. A nonplatinum combination in metastatic transitional cell carcinoma. American Journal of Clinical Oncology. 2005;28(2):114–118. Reason: includes patients treated with 1st line chemo . [PubMed: 15803002]
- Takahashi S, et al. Combination chemotherapy of docetaxel, ifosfamide and cisplatin (DIP) in patients with metastatic urothelial cancer: a preliminary report. Japanese Journal of Clinical Oncology. 2005;35(2):79–83. Reason: includes patients treated with 1st line chemo . [PubMed: 15709091]
- Gallagher DJ, Al-Ahmadie H, Ostrovnaya I, Gerst SR, Regazzi A, Garcia-Grossman I, Riches J, Gounder SK, Flaherty AM, Trout A, Milowsky MI, Bajorin DF. Sunitinib in urothelial cancer: clinical, pharmacokinetic, and immunohistochemical study of predictors of response. Eur.Urol. 2011;60(2):344–349. Reason: same study as Gallagher 2010 . [PubMed: 21645967]
- Stordal B. Oxaliplatin for the treatment of cisplatin-resistant cancer: A systematic review. Cancer Treat.Rev. 2007;33(4):347–357. Reason: includes studies of non urothelial cancers . [PubMed: 17383100]
- Grimm MO, Machiels JP, Wulfing C, Richel D, Treiber U, de Groot M, Beuzeboc P, Farrell J, Colman J, Colman J, El-Hariry I. A single arm, multicentre, open-label phase II study of orally administered lapatinib (GW572016) as single-agent, second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. AnnOncol. 2005;16:41. Reason: same study as Wulfing 2009, abstract only .
- Font A, Esteban E, Carles J, Climent MA, Gonzalez-Larriba JL, Berrocal A, Bellmunt J, Garcia-Ribas I, Marfa X, Fabregat X. Gemcitabine and oxaliplatin combination: A multicenter phase II trial in unfit patients with locally advanced or metastatic urothelial cancer. J.Clin.Oncol. 2004;22(14):392S. Reason: unclear if 2nd line chemotherapy, abstract only . [PubMed: 17693649]
- Bajorin DF. Paclitaxel/cisplatin/ifosfamide in advanced bladder cancer. Semin.Oncol. 1999;26(1):139. Reason: unclear if 2nd line chemotherapy, abstract only .
- Theodore C. Multicenter phase II study of sunitinib in patients with transitional cell carcinoma of the urothelium (TCCU) who failed or progressed after first line chemotherapy (CT) for locally advanced or metastatic disease. Preliminary results. Ann.Oncol. 2010.:viii281. Conference (var.pagings) Reason: abstract only, no overall survival data, accrual stopped as no responses to treatment .
- Bradley D, Daignault S, Smith DC, Nanus D, Tagawa S, Stadler WM, Garcia J, Dreicer Hawary R, Al M, Hussain M. Maintenance sunitinib postchemotherapy (CT) in patients (pts) with advanced urothelial carcinoma (UC): A randomized placebo controlled phase II trial. Journal of ClinicalOncology. 2009;27:252. [ abstract no. 5073 ] Reason: abstract only, maintenance treatment not relevant to PICO .
- Cervera Grau JM. Pemetrexed as rescue in urothelial carcinoma of the bladder. Preliminar results from a follow-up in arco del mediterraneo group. Ann.Oncol. 2008. Conference (ESMO):var. Reason: abstract only, includes 1st line treatment .
- Grau J. Cervera. Diverse long-time progression-free survival (PFS) and overall survival (OS), based on metastasis location, in metastatic urothelial carcinoma (MUC) patients treated with pemetexed (P) in monotherapy: Results from a longer follow-up of Arco del Mediterraneo Group. J.Clin.Oncol. 2010;15 Conference (var.pagings) Reason: abstract only, includes 1st line treatment .
- Rozzi A, et al. Weekly Paclitaxel as Third-line Chemotherapy in Patients With Metastatic Transitional Cell Carcinoma of Urothelial Tract: Results of a Phase II Study. European Journal of Cancer. 2011;47:S518–S518. Reason: abstract only, 3rd line chemotherapy . [PubMed: 21116878]
- Pu YS, et al. Gemcitabine and ifosfamide (GI) as a second-line systemic chemotherapy for cisplatin-failed advanced urothelial carcinoma (UC). Journal of Clinical Oncology. 2004;22(14):441S–441S. Reason; same study as Lin 2007, abstract only .
- Martinez P, et al. Docetaxel activity in second line treatment for urothelial carcinoma: a retrospective analysis. Ejc Supplements. 2009;7(2):448–448. Reason; retrospective analysis, abstract only .
- Chaudhary UB, et al. Gemcitabine and irinotecan combination in patients with metastatic bladder cancer: A phase II trial. Journal of Clinical Oncology. 2005;23(16):441S–441S. Reason: immature data, abstract only .
- Kawai K, et al. Maintenance chemotherapy with gemcitabine and paclitaxel for M-VAC refractory metastatic transitional cell carcinoma. Journal of Urology. 2005;173(4):360–360. Reason: abstract only, appears same study as Takahashi 2006 .
- Kaya AO, et al. Paclitaxel plus Doxorubicin Chemotherapy as Second-Line Therapy in Patients with Advanced Urothelial Carcinoma Pretreated with Platinum plus Gemcitabine Chemotherapy. Onkologie. 2012;35(10):576–580. Reason: retrospective analysis, doxorubicin not in PICO . [PubMed: 23038228]
- Tanji N, et al. Long-term results of combined chemotherapy with gemcitabine and cisplatin for metastatic urothelial carcinomas. International Journal of Clinical Oncology. 2010;15(4):369–375. Reason: retrospective analysis . [PubMed: 20340038]
- Lee JL, et al. Phase II study of a cremophor-free, polymeric micelle formulation of paclitaxel for patients with advanced urothelial cancer previously treated with gemcitabine and platinum. Investigational New Drugs. 2012;30(5):1984–1990. Reason; intervention not relevant to PICO . [PubMed: 22012004]
- Soga N. Third-line gemcitabine monotherapy for platinum-resistant advanced urothelial cancer. International Journal of Clinical Oncology. 2010;15(4):376–381. Reason: intervention not relevant to PICO (3rd line chemotherapy) [PubMed: 20333430]
Evidence tables
Download PDF (270K)
5.2. Managing symptoms of locally advanced or metastatic bladder cancer
6.2.1. Bladder symptoms
Review question: What is the optimal pelvic radiotherapy regimen for patients with incurable locally advanced or metastatic bladder cancer?
Rationale
Radiotherapy can be used to help patients with symptoms of incurable bladder cancer. It is most commonly used to treat bleeding from the bladder or pain from the bladder cancer itself or sites of spread. Radiotherapy is also used to improve local control rates in patients with advanced pelvic disease. Treatment is usually given between 1 and 10 fractions as an outpatient. Side-effects are related to the area treated but are usually well-tolerated. For example, bladder radiotherapy can result in short term urinary frequency and discomfort or diarrhoea and nausea. These symptoms can be easily managed using appropriate medication. There is little evidence of differences in toxicity and outcome of patients of different gender or age. The total dose and fractionation of radiotherapy varies across the UK. Some clinicians deliver palliative radiotherapy at the time of diagnosis whilst others delay treatment until the patient becomes symptomatic. There have been a limited number of randomised control trials in this topic.
This review should establish the optimum radiotherapy regime which benefits patients with incurable bladder cancer by establishing which doses and fractionation maximise symptom control and local disease control rates. The timing of radiotherapy (immediate at the time of diagnosis or delayed until patient is symptomatic) should also be evaluated.
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with incurable locally advanced or metastatic bladder cancer | Palliative pelvic radiotherapy | Dose/fractionation, timing to treat, duration of treatment |
|
METHODS
Information sources
A literature search was performed by the information specialist (DM)
Selection of studies
The information specialist (DM) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. The full articles were then obtained for potentially relevant studies and checked against the inclusion criteria. Comparative studies and palliative radiotherapy series were selected for this review question.
Data synthesis
Data was extracted into RevMan and risk ratios were calculated were possible. No meta-analysis was possible for this review question.
RESULTS
Study quality and results
The included evidence is summarised in Tables 171-173.
Table 171
GRADE evidence profile: Palliative radiotherapy – 35Gy in 10 fractions versus 21Gy in 3 fractions.
Table 172
GRADE evidence profile: Hypofractionated radiotherapy versus conventional palliative radiotherapy.
Table 173
GRADE evidence profile: Palliative radiotherapy for bladder cancer (observational studies).
Evidence statements
Moderate quality evidence about the relative effectiveness of two hypofractionated radiotherapy schedules (35 Gy in 10 fractions over two weeks versus 21 Gy in 3 fractions over one week) for local symptom control of muscle invasive bladder cancer came from one randomised trial (Duchesne et al., 2000). 500 patients were randomised with three month follow-up data available in 272 patients. Overall symptom improvement, defined as improvement of at least one symptom by one grade without worsening another symptom, was 71% in those receiving 35-Gy compared with 64% in the 21-Gy arm, though there is uncertainty of a difference between treatments (absolute improvement 3%, 95% CI -6% to 12%). Comparing the 35 Gy group with the 21 Gy group for patients with specific pre-treatment symptoms, urinary frequency resolved in 43% and 42%, respectively, nocturia in 51% and 35%, haematuria in 58% and 61%, and dysuria in 47% and 49%. Median survival was 7.5 months in both groups. Two-thirds of participants reported that quality of life symptom scores were either unchanged or improved by the end of treatment and at three months after treatment.
One observational study (Srinivasan et al., 1994) provided low quality evidence about the relative effectiveness of hypofractionated (two-fraction) radiotherapy and conventional palliative radiotherapy in 41 patients selected by performance status. 59% of those receiving two-fraction radiotherapy had clearance of haematuria compared to 16% of those receiving conventional palliation (RR 3.74, 95% CI 1.25 to 11.19). Pain improved in 73% of those treated with two-fraction radiotherapy compared to 37% of those treated with conventional palliation (RR 1.97, 95% CI 1.04 to 3.75). All patients died during follow-up. Mean survival was 9.77 and 14.47 months in the hypofractionated and conventional radiotherapy groups respectively.
Very low quality evidence was reported from seven observational studies using various palliative radiotherapy regimens. Median survival ranged from six to nine months across studies. Complete palliation of symptoms was achieved in 51% of 65 elderly patients treated with 30 Gy in five fractions on a weekly basis, although 28 patients experienced transient worsening of their urinary symptoms with eight requiring hospital admission due to toxicities (McLaren et al., 1997). Jose et al. (1999) reported a similar radiotherapy schedule with control of haematuria in 50%, frequency in 63%, dysuria 38%, and nocturia 5%. This study also reported toxicity rates of 36% for acute bowel and 63% for acute bladder toxicity. One study of short-term radiotherapy (7Gy 3 times or 5Gy 4 times) reported that none of the 17 patients with severe local symptoms improved after radiotherapy, although improvement was difficult to assess as 10 of these patients died within four months (Holmang et al., 1995). Haematuria was present in 14 patients but it continued in only two after radiotherapy. Another study of short-term radiotherapy (Wijkstrom et al., 1991) reported an improvement in tumour associated symptoms in 75/162 (46%) patients, although 42% had various minor acute side effects and over half the population were treated for tumours considered to be curable. Five-year survival in patients considered to be curable was 21%, compared to 6% in patients treated for bleeding and 0% for patients with other local symptoms.
References to included studies
- Duchesne GM, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. International Journal of Radiation Oncology, Biology, Physics. 2000;47(2):379–388. [PubMed: 10802363]
- Holmang S, Borghede G. Early complications and survival following short-term palliative radiotherapy in invasive bladder carcinoma. Journal of Urology. 1996;155(1):100–102. [PubMed: 7490801]
- Jose CC, et al. Hypofractionated radiotherapy for patients with carcinoma of the bladder. Clinical Oncology (Royal College of Radiologists). 1999;11(5):330–333. [PubMed: 10591821]
- Kouloulias V, et al. Evaluation of Acute Toxicity and Symptoms Palliation in a Hypofractionated Weekly Schedule of External Radiotherapy for Elderly Patients with Muscular Invasive Bladder Cancer. International Braz J Urol. 2013;39(1):77–82. [PubMed: 23489500]
- McLaren DB, Morrey D, Mason MD. Hypofractionated radiotherapy for muscle invasive bladder cancer in the elderly. Radiotherapy and Oncology. 1997;43(2):171–174. [PubMed: 9192963]
- Salminen E. Unconventional fractionation for palliative radiotherapy of urinary bladder cancer. A retrospective review of 94 patients. Acta Oncologica. 1992;31(4):449–454. [PubMed: 1378746]
- Saunders D, Kiltie A. Palliative radiotherapy for bladder cancer: The Leeds teaching hospitals experience. Radiotherapy and Oncology. 2006;81:S532–S532.
- Spagnoletti G, et al. Palliative radiotherapy for bladder cancer: A small retrospective study. Anticancer Research. 2010;30(4):1515.
- Srinivasan V, Brown CH, Turner AG. A comparison of two radiotherapy regimens for the treatment of symptoms from advanced bladder cancer. Clinical Oncology (Royal College of Radiologists). 1994;6(1):11–13. [PubMed: 7513538]
- Wijkstrom H, et al. Short-term radiotherapy as palliative treatment in patients with transitional cell bladder cancer. British Journal of Urology. 1991;67(1):74–78. [PubMed: 1704277]
References to excluded studies (with reasons for exclusion)
- Cameron MG, et al. Patient reported outcomes of symptoms and quality of life among cancer patients treated with palliative pelvic radiation: a pilot study. BMC Research Notes. 2011;4:252. Reason: pilot study (n(=)22), mostly prostate cancer, outcomes not relevant to PICO . [PMC free article: PMC3154166] [PubMed: 21777440]
- Caravatta L, et al. Short-course accelerated radiotherapy in palliative treatment of advanced pelvic malignancies: a phase I study. International Journal of Radiation Oncology, Biology, Physics. 2012;83(5):e627–e631. Reason: mostly gynaecologic cancers, phase I study (n(=)27) [PubMed: 22580117]
- Yamaguchi S, et al. Palliative radiotherapy in patients with a poor performance status: The palliative effect is correlated with prolongation of the survival time. Radiation Oncology. 2013;8(1) Reason: not pelvic RT, mostly lung cancer patients . [PMC free article: PMC3707862] [PubMed: 23829540]
- Van WN, et al. Determination of margins for pelvic lymph nodes for the treatment of bladder cancer. International Journal of Radiation Oncology Biology Physics. 2011;81(2 SUPPL. 1):S449–S450. Reason: outcomes not relevant to PICO .
- Toscano G, et al. Role of radical radiotherapy (RRT) in the treatment of inoperable invasive bladder cancer in the elderly. European Journal of Cancer. 1997;33:140–140. Reason: unclear if relevant to PICO, abstract only .
- Ok J-H, Meyers FJ, Evans CP. Medical and surgical palliative care of patients with urological malignancies. Journal of Urology. 2005;174(4 I):1177–1182. Reason: Expert review . [PubMed: 16145365]
- Nishioka K, et al. Organ-conserving definitive radiotherapy for locally advanced bladder carcinomawith image-guided local boost. International Journal of Radiation Oncology Biology Physics. 2011;81(2 SUPPL. 1):S449. Reason: abstract only, insufficient information to include .
- Moonen L, et al. A feasibility study of accelerated fractionation in radiotherapy of carcinoma of the urinary bladder. International Journal of Radiation Oncology, Biology, Physics. 1997;37(3):537–542. Reason: population not relevant to PICO (radical radiotherapy) [PubMed: 9112450]
- Lutz ST, et al. A review of hypofractionated palliative radiotherapy. Cancer. 2007;109(8):1462–1470. Reason: expert review . [PubMed: 17330854]
- Hoskin PJ. Optimisation of palliative radiotherapy. European Journal of Cancer, Supplement. 2007;5(5):380–382. Reason: expert review .
- Harris V, Warren-Oseni K, Huddart R. Radiotherapy planning study comparing VMAT, IMRT and 3D-CRT in the treatment of bladder and pelvic lymph nodes. Radiotherapy and Oncology. 2012;103:S589–S590. Reason: abstract only, unclear if relevant to PICO .
- Fetscher S, Schmielau J, Schulze-Seemann W. Five-year, disease-free survival after repeat palliative multimodality therapy in a patient with recurrent metastastic bladder cancer. The scientific world journal. 2007;7:1736–1742. Reason: case study . [PMC free article: PMC5900847] [PubMed: 17982569]
- De SM, et al. Combined chemo-radiotherapy with gemcitabine in patients with locally advanced inoperable transitional cell carcinoma (TCC) of the urinary bladder and/or in patients ineligible for surgery: Results of a phase I trial. Annals of Oncology. 2010;21:viii274. Reason: not relevant to PICO (chemo-radiotherapy) [PubMed: 24936582]
- Carillio G, et al. A phase I trial of conformal radiotherapy plus gemcitabine for the treatment of locally advanced or relapsed bladder cancer. Annals of Oncology. 2006;17:XI77–XI77. Reason: not relevant to PICO (chemo-radiotherapy)
- Graham JD, et al. Palliative radiotherapy for muscle invasive bladder cancer: Final results of a prospective randomised trial of two radiotherapy schedules. British Journal of Cancer. 2000;83:27–27. Reason: duplicate of Duchesne, abstract only .
- vom Dorp F, Borgermann C, Rubben H. Palliative therapy concepts for patients with urothelial cancer of the urinary bladder. Urologe. 2007;46(1):54–55. Reason: foreign language . [PubMed: 17203266]
- Fossa SD. Pelvic Palliation Radiotherapy of Advanced Bladder-Cancer. International Journal of Radiation Oncology Biology Physics. 1991;20(6):1379–1379. Reason: editorial . [PubMed: 1710614]
- Wesson MF. Radiation-Therapy in Regionally Advanced Bladder-Cancer. Urologic Clinics of North America. 1992;19(4):725–734. Reason: expert review on curative radiotherapy . [PubMed: 1441028]
- Spanos J, et al. Phase II study of multiple daily fractionations in the palliation of advanced pelvic malignancies: Preliminary report of RTOG 8502. International Journal of Radiation Oncology Biology Physics. 1989;17(3):659–661. Reason: mostly gynaecologic and colorectal malignancies, results not reported separately . [PubMed: 2476426]
- Vitale V. When to implement radiotherapy. Tumori. 2003:S113–S113. Reason: foreign language, abstract only .
- Zygogianni A, et al. A weekly hypofractionated radiotherapeutic schedule for bladder carcinoma in elderly patients: local response, acute and late toxicity, dosimetric parameters and pain relief. Journal of B.U.On. 2013;18(2):407–412. Reason: appears to be same study as Kouloulias (2013) [PubMed: 23818353]
Evidence tables
Download PDF (385K)
5.2.2. Loin pain and symptoms of renal failure
Review question: What is the best way to manage cancer related ureteric obstruction in patients with bladder cancer?
Rationale
In patients with locally advanced bladder cancer, with or without metastases, the tumour can sometimes obstruct one or both ureters (the tubes connecting the kidneys to the bladder). If only one kidney is obstructed, the opposite kidney can usually maintain normal kidney function. Here the decision to intervene is often based on whether the patient has symptoms such as loin pain or whether optimal kidney function is essential e.g to enable safe administration of systemic chemotherapy. However, if both kidneys are obstructed then urine cannot pass and the patient will develop kidney failure which if untreated is fatal. Fortunately this type of presentation is relatively uncommon. Historically these patients were often managed conservatively with no intervention and this is still one option. However the obstruction can be relieved either by inserting a stent (an internal plastic drainage tube) under general anaesthetic or by a radiologist inserting a nephrostomy (a plastic drainage tube which comes out through the skin and drains into an external bag).
There are no current guidelines or good quality randomised trials in this area and treatment is often based on opinion or local resources leading to widespread variations in practice across the UK.
Not treating the obstruction is uniformly fatal and in the last decade, as a result of a greater public awareness of issues surrounding end of life care, is often unacceptable to patients and their carers. The benefit of surgical insertion of a stent is that the patient does not have an external urine bag. It may also be possible to remove some of the obstructing tumour. However, the tumour is often very advanced making it impossible to identify the ureteric openings to insert the stent and the patient who is often very sick from kidney failure will have been exposed to the risks of an anaesthetic but with an unsuccessful outcome. Even with successful stenting the obstructing tumour can prevent adequate urine drainage necessitating subsequent nephrostomy drainage.
The benefit of a nephrostomy insertion is that the procedure can be carried out under light sedation, if necessary in a ward setting (e.g ITU) and improvement in kidney function is independent of the tumour obstruction further down the ureter. The main disadvantage is that if the patient's blood clotting is deranged as is often the case in kidney failure, then nephrostomy insertion is potentially dangerous due to the risks of causing internal bleeding. It also requires an experienced interventional radiologist which may not be available particularly out of hours or in a small DGH.
The benefits and harms of doing nothing or intervention with either a stent or a nephrostomy should be outlined. Recommendations should also cover whether the obstruction affects one or both kidneys and, in the latter group, whether or not the patient has reached end of life care.
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with cancer related ureteric obstruction | Urinary stent Surgery - urinary diversion Percutaneous nephrostomy | Best supportive care Each other |
|
METHODS
Information sources
A literature search was performed by the information specialist (EH).
Selection of studies
The information specialist (EH) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. The full articles were then obtained for potentially relevant studies and checked against the inclusion criteria. Studies were also considered if they included patients with ureteric obstruction caused by malignancies other than primary bladder cancer. Studies must include at least 50 patients with malignant obstruction to be included.
Data synthesis
Data was extracted into GRADE. No meta-analysis was possible for this review question. A narrative summary of the evidence is presented.
RESULTS
Study quality and results
30 retrospective case series studies and one prospective quality of life study were identified for this evidence review. Evidence is summarised in Tables 174-182.
Table 174
GRADE evidence profile: Open nephrostomy, percutaneous nephrostomy, retrograde stents for malignant obstructions.
Table 175
GRADE evidence profile: Retrograde stents for malignant obstructions.
Table 176
GRADE evidence profile: Percutaneous nephrostomy for malignant obstructions secondary to bladder cancer.
Table 177
GRADE evidence profile: Percutaneous nephrostomy for malignant obstructions.
Table 178
GRADE evidence profile: Retrograde stent versus percutaneous nephrostomy for malignant obstructions.
Table 179
GRADE evidence profile: Subcutaneous nephro-vesical/ nephro-cutaneous bypass for malignant obstructions.
Narrative summary of the evidence
Open nephrostomy and ureteral stents
Very low quality evidence was provided by one retrospective review of open surgical techniques and non operative urinary diversion for malignant ureteral obstructions (Zadra, 1987). After 1981, no patients required open nephrostomy.
Improvement of renal function
60/88 patients undergoing bilateral procedures had normal renal function after diversion, although this outcome was not reported separately for each procedure.
Treatment-related morbidity
The overall complication rate was highest for open nephrostomy (57%, including dislodgement and infection/sepsis). Complication rates for percutaneous nephrostomy (PCN) and retrograde stents were 25% (dislodgement, blockage, infection) and 19% (dislodgement, blockage), respectively. Prostate and bladder tumours were the most difficult to divert by retrograde stents because the ureteral orifices were more difficult to see or were invaded by the tumour.
Overall survival
The average survival of patients undergoing open nephrostomy was 3.8 months. In the 38 patients who died after retrograde stenting or PCN, the average overall survival was 6.5 months.
No evidence was available about the impact of open nephrostomy and ureteral stents on subsequent chemotherapy, subsequent cystectomy, symptom relief or health related quality of life
Retrograde stents
Five retrospective series provided evidence about retrograde stents for malignant obstructions. Evidence for all outcomes was of very low quality. In Shekarriz (1999) patients underwent PCN or retrograde stenting, but it is not clear how many participants had each procedure and the outcomes were not reported separately per procedure.
Improvement of renal function
Three studies (313 patients) reported that average serum creatinine levels decreased after retrograde stent placement by 34% to 57% across studies (Shekarriz 1999; Ganatra 2005; Kamiyama 2011).
Symptom relief
One study reported that 50/90 (56%) participants showed resolution of hydronephrosis and flank pain or renal failure after stent placement (Chung 2004).
Treatment-related morbidity
A 65.6% complication rate was reported by three studies (302 participants), including catheter complications, hematuria and UTI (Shekarriz 1999; Ganatra 2005; Kamiyama 2011). Izumi (2011) did not report treatment-related morbidity but reported that there were no major complications with retrograde stent placement.
Overall survival
Four studies (374 participants) reported an average length of overall survival, ranging from 2.2 months to 11.1 months. Izumi (2011) reported a median survival time of 7.6 months, with Scr before stent placement of 1.2 mg/dl or greater, no treatment after stenting, and cancer group (especially non-gynaecologic cancers) were prognostic factors for unfavourable overall survival. Gastrointestinal (GI) cancer was associated with a shorter overall survival. 57% of the population in Kamiyama (2011) had primary GI cancer, and this study had the shortest median survival of 2.2 months (range 1-546 days). Type of cancer did not predict stent failure in one study, although 56% of participants with invasion into the bladder on cystoscopy progressed to PCN referral (Ganatra 2005).
Subsequent chemotherapy
26/61 (42.6%) of patients received chemotherapy after treatment of malignant obstruction (Izumi 2011). In total 39/61 (64%) received some form of treatment for cancer after stent placement.
Percutaneous nephrostomy for obstruction secondary to bladder cancer
Three studies (132 participants) reported very low quality evidence on percutaneous nephrostomy for the treatment of ureteric obstruction secondary to bladder cancer.
Improvement of renal function
One study (23 participants) reported that 18/23 (83%) patients improved to normal renal function after PCN (Ekici 2001).
Symptom relief
No evidence available
Treatment-related morbidity
The overall complication rate reported by three studies (Ekici 2003; Gupta 2007; El-Tabey 2005) was 20.2% (22/109), including PCN tube related complications and hematuria.
Overall survival
Three studies reported the rate of overall survival at follow-up, with 37/97 (38%) patients alive at mean follow-up ranging from 16-34 months. Median overall survival was 4.9 months (range 1-14) in one study (Ekici, 2001).
Subsequent chemotherapy
One study reported that 11/23 (48%) of patients underwent chemotherapy after PCN (Ekici, 2001).
Subsequent cystectomy
Three studies reported that 66/142 (46.5%) patients underwent cystectomy after PCN.
Health-related quality of life
No evidence available
Percutaneous nephrostomy for malignant obstruction
14 studies provided very low quality evidence on PCN for malignant obstructions.
Improvement of renal function
Six studies (795 patients) providing very low quality evidence reported a decrease in average serum creatinine levels after the PCN procedure. Two studies reported that 208/241 (86.3%) patients returned to normal renal function or showed a significant improvement after the procedure. In Pappas (2000), patients with gynaecological malignancy showed the best improvement rates.
Symptom relief
Relief of obstruction was reported in 151/248 (61%) patients (2 studies).
Treatment-related morbidity
11 studies reported an overall complication rate of 29% (447/1523).
Overall survival
Average overall survival was reported by eleven studies with length of survival ranging from 3.2 to 12.2 months across studies.
Subsequent chemotherapy
One study reported that 27 out of 38 patients (71%) underwent chemotherapy and/or radiotherapy after PCN (Meyer 1980).
Subsequent cystectomy
One study reported that 4 out of the 29 patients with bladder cancer in the cohort underwent cystectomy after nephrostomy (Fallon 1980).
Health-related quality of life
One study (270 patients) measured quality of life with the EORTC-QLQ and reported that there was no improvement in scores over the study period (Aravantinos 2007).
Percutaneous nephrostomy and retrograde stents for malignant obstruction
Seven studies reported the outcomes of patients who received PCN and those who received retrograde stents for malignant obstructions. All outcomes were assessed as being of very low quality.
Improvement of renal function
Three studies reported serum creatinine levels before and after interventions for malignant obstruction. Ku (2004) reported that both ureteral stenting and PCN resulted in a decrease of serum creatinine, with no significant difference between groups. Kanou (2007) reported that renal function improved in all patients and the average serum creatinine decreased after urinary diversion. Renal function was not reported separately for patients undergoing urinary stenting or nephrostomy. One study reported that serum creatinine increased in all patients, with a smaller elevation of creatinine levels in the PCN group (0.21 mg/dL) than in the stent group (0.78) (Chang 2012).
Symptom relief
One study of 110 patients reported that residual hydronephrosis after diversion was more common in the stent group than the PCN group (65% versus 27%) (Chang 2012).
Treatment-related morbidity
Four studies reported complications of PCN (n=218) and ureteral stents (n=156). Similar rates of complications were reported with ureteral stents (28.8%) and PCN (30.3%). A further study (Chang 2012) reported that the stent group had more frequent UTI, including urosepsis and pyelonphritis, than the PCN group, although this difference was non-significant.
Overall survival
Two studies reported overall survival in patients who underwent stenting and in those who underwent PCN. Average overall survival was 5.6 and 9.2 months for ureteral stents and 5.9 and 6.5 months for PCN. A further study reported an overall survival of 6.1 months for all patients regardless of the intervention received for ureteric obstruction. Multivariate analyses by Wong (2007) revealed that the presence of metastases and a diagnosis of malignant obstruction in previously established malignancy were independent prognostic factors for inferior overall survival.
Subsequent chemotherapy
One study reported that 21% (11/52) of patients were treated with chemotherapy after successful drainage of the kidneys. It is not reported which intervention these patients received (Hubner 1993).
Subsequent cystectomy
One patient out of 30 with bladder cancer had a total cystectomy with urinary diversion for muscle-invasive disease after relief of obstruction in Chitale (2002).
Health-related quality of life
One study reported that responses to quality of life surveys were not significantly different for patients receiving nephrostomy tubes (n=16), double-J stents (n=15) or nephroureteral stents (NUS, n=15). Patients who had double J stents reported more pain, dysuria, and urinary frequency, compared with nephrostomy tubes and NUS at 30 and 90 days after placement.
Subcutaneous nephro-vesical/ nephro-cutaneous bypass for malignant obstructions
One study of 52 patients with metastatic disease undergoing palliative subcutaneous bypass was reported as a conference abstract only (Schmidbauer 2009). All outcomes were assessed as very low quality.
Improvement of renal function
Serum creatinine levels decreased from a mean of 6.1 to 1.55 mg/%.
Symptom relief
Preoperative hydronephrosis was completely eliminated in 80.8% of the renal units and was dramatically reduced in the remaining units.
Treatment-related morbidity
15/52 (28.8%) patients had UTI. 11 patients had a single and 4 patients recurrent UTI which resolved under antibiotics.
Overall survival
After a mean follow-up of 12.0 months (range 2-57) all but 4 patients had died from their progressive metastatic disease.
Health-related quality of life
On a range of 1 (very poor) to 10 (excellent), mean quality of life score was 3.6 (range 0-6) pre-operatively, and 7.8 (range 5-9) post-operatively.
No evidence available about subsequent chemotherapy and subsequent cystectomy
Evidence statements
Very low quality evidence was identified from 30 retrospective observational studies. All studies report an improvement of renal function and symptom relief in a majority of patients after PCN or stent placement. Seven studies reported the comparative outcomes of patients who received PCN and those who received retrograde stents for malignant obstructions. Ku et al. (2004) reported that both ureteral stenting and PCN resulted in a decrease of serum creatinine, with no significant difference between groups. One study reported that serum creatinine increased in all patients (n=110), with a smaller elevation of creatinine levels in the PCN group than in the stent group (Chang et al. 2012). This study also reported that residual hydronephrosis after diversion was more common in the stent group than the PCN group (65% versus 27%).
Four studies reported complications of PCN (n=218) and ureteral stents (n=156). Similar rates of complications were reported with ureteral stents (28.8%) and PCN (30.3%). A further study (Chang et al. 2012) reported that the stent group had more frequent UTI, including urosepsis and pyelonphritis, than the PCN group, although this difference was non-significant.
Two studies reported overall survival in patients who underwent stenting and in those who underwent PCN (Kanou et al., 2007; Wong et al., 2007). Average overall survival was 5.6 and 9.2 months for ureteral stents and 5.9 and 6.5 months for PCN.
One study reported that 21% (11/52) of patients were treated with chemotherapy after successful drainage of the kidneys. It is not reported which intervention these patients received (Hubner et al. 1993). In one study, 1/30 patients with bladder cancer had a total cystectomy with urinary diversion for muscle-invasive disease after relief of obstruction (Chitale et al., 2002).
One study reported that responses to quality of life surveys were not significantly different for patients receiving nephrostomy tubes (n=16), double-J stents (n=15) or nephroureteral stents (NUS, n=15). Patients who had double-J stents reported more pain, dysuria, and urinary frequency, compared with nephrostomy tubes and NUS at 30 and 90 days after placement (Monsky et al., 2013).
References to included studies
- Aravantinos E, et al. Percutaneous nephrostomy in patients with tumors of advanced stage: Treatment dilemmas and impact on clinical course and quality of life. Journal of Endourology. 2007;21(11):1297–1302. [PubMed: 18042018]
- Carrafiello G, et al. Complications of percutaneous nephrostomy in the treatment of malignant ureteral obstructions: single-centre review. Radiologia Medica. 2006;111(4):562–571. [PubMed: 16779542]
- Chang HC. Comparison between the use of percutaneous nephrostomy and internal ureteral stenting in the management of long-term ureteral obstructions. Urological Science. 2012;23(3):82–84.
- Chitale SV, et al. The management of ureteric obstruction secondary to malignant pelvic disease. Clinical Radiology. 2002;57(12):1118–1121. [PubMed: 12475538]
- Chung SY, et al. 15-year experience with the management of extrinsic ureteral obstruction with indwelling ureteral stents. Journal of Urology. 2004;172(2):592–595. [PubMed: 15247739]
- Ekici S, Sahin A, Ozen H. Percutaneous nephrostomy in the management of malignant ureteral obstruction secondary to bladder cancer. Journal of Endourology. 2001;15(8):827–829. [PubMed: 11724123]
- El-Tabey NA, et al. Bladder cancer with obstructive uremia: oncologic outcome after definitive surgical management. Urology. 2005;66(3):531–535. [PubMed: 16140072]
- Fallon B, Olney L, Culp DA. Nephrostomy in cancer patients: to do or not to do? British Journal of Urology. 1980;52(4):237–242. [PubMed: 7426987]
- Ganatra AM, Loughlin KR. The management of malignant ureteral obstruction treated with ureteral stents. Journal of Urology. 2005;174(6):2125–2128. [PubMed: 16280741]
- Gupta NP, et al. Oncological and functional outcome of radical cystectomy in patients with bladder cancer and obstructive uropathy. Journal of Urology. 2007;178(4 Pt 1):1206–1211. [PubMed: 17698145]
- Hubner WA. Hydronephrosis in malignant tumors: Rationale and efficiency of endo-urological diversions. European Journal of Surgical Oncology. 1993;19(1):27–32. [PubMed: 8436237]
- Ishioka J, et al. Prognostic model for predicting survival after palliative urinary diversion for ureteral obstruction: analysis of 140 cases. Journal of Urology. 2008;180(2):618–621. [PubMed: 18554655]
- Izumi K, et al. Current outcome of patients with ureteral stents for the management of malignant ureteral obstruction. Journal of Urology. 2011;185(2):556–561. [PubMed: 21168872]
- Kamiyama Y, et al. Stent failure in the management of malignant extrinsic ureteral obstruction: risk factors. International Journal of Urology. 2011;18(5):379–382. [PubMed: 21518020]
- Kanou T, et al. Management of extrinsic malignant ureteral obstruction with urinary diversion. International Journal of Urology. 2007;14(8):689–692. [PubMed: 17681056]
- Kinn AC, Ohlsen H. Percutaneous nephrostomy--a retrospective study focused on palliative indications. APMIS. 2003;(109) Supplementum:66–70. [PubMed: 12874953]
- Ku JH, et al. Percutaneous nephrostomy versus indwelling ureteral stents in the management of extrinsic ureteral obstruction in advanced malignancies: are there differences? Urology. 2004;64(5):895–899. [PubMed: 15533473]
- Lau MW, et al. Urinary tract obstruction and nephrostomy drainage in pelvic malignant disease. British Journal of Urology. 1995;76(5):565–569. [PubMed: 8535673]
- Liatsikos EN, et al. Ureteral metal stents: 10-year experience with malignant ureteral obstruction treatment. Journal of Urology. 2009;182(6):2613–2617. [Review] [16 refs] [PubMed: 19836807]
- Lienert A, Ing A, Mark S. Prognostic factors in malignant ureteric obstruction. BJU International. 2009;104(7):938–941. [PubMed: 19338533]
- Meyer JE, Yatsuhashi M, Green TH Jr. Palliative urinary diversion in patients with advanced pelvic malignancy. Cancer. 1980;45(10):2698–2701. [PubMed: 6155198]
- Monsky WL, et al. Quality-of-life assessment after palliative interventions to manage malignant ureteral obstruction. Cardiovascular & Interventional Radiology. 2013;36(5):1355–1363. [PMC free article: PMC4601638] [PubMed: 23404519]
- Pappas P, et al. Role of percutaneous urinary diversion in malignant and benign obstructive uropathy. Journal of Endourology. 2000;14(5):401–405. [PubMed: 10958560]
- Radecka E, Magnusson M, Magnusson A. Survival time and period of catheterization in patients treated with percutaneous nephrostomy for urinary obstruction due to malignancy. Acta Radiologica. 2006;47(3):328–331. [PubMed: 16613316]
- Schmidbauer J. Palliative urinary diversion by subcutaneous nephro-vesical/nephro-cutaneous bypass in end-stage malignant disease. Journal of Urology. 2009. Conference(AUA): var.
- Sheikh N, et al. An audit of long term follow-up of antegrade ureteric stenting as a procedure of choice for the management of obstructive uropathy in pelvic malignancies. BJU International. 2007;99:31–31.
- Shekarriz B, et al. Outcome of palliative urinary diversion in the treatment of advanced malignancies. Cancer. 1999;85(4):998–1003. [PubMed: 10091780]
- Vehmas T, et al. Results and complications of percutaneous nephrostomy. Annals of Clinical Research. 1988;20(6):423–427. [PubMed: 3218915]
- Watkinson AF, et al. The role of percutaneous nephrostomy in malignant urinary tract obstruction. Clinical Radiology. 1993;47(1):32–35. [PubMed: 8428414]
- Wong LM, et al. Malignant ureteral obstruction: outcomes after intervention. Have things changed? Journal of Urology. 2007;178(1):178–183. [PubMed: 17499300]
- Zadra JA, et al. Nonoperative urinary diversion for malignant ureteral obstruction. Cancer. 1987;60(6):1353–1357. [PubMed: 3621117]
References to excluded studies (with reasons for exclusion)
- Hoshino K, et al. Preoperative Hydronephrosis: Independent Predictor for Changes in Renal Function Following Nephroureterectomy. Japanese Journal of Clinical Oncology. 2012;42(3):202–207. [PubMed: 22246718]
- Carrafiello G, et al. Direct primary or secondary percutaneous ureteral stenting: what is the most compliant option in patients with malignant ureteral obstructions? Cardiovascular & Interventional Radiology. 2007;30(5):974–980. [PubMed: 17468910]
- Richter F, et al. Endourologic management of malignant ureteral strictures. Journal of Endourology. 2000;14(7):583–587. [PubMed: 11030541]
- Oh KJ, et al. Management of extrinsic malignant ureteral obstruction in nongenitourinary malignancies: Retrograde internal ureteral stenting vs percutaneous nephrostomy. Journal of Endourology. 2007;21:A257–A258.
- Hwang EC. Predicting factors for stent failure free survival in patients with a malignant ureteral obstruction managed with ureteral stents. Journal of Endourology. 2012.:A427–A428. Conference(var.pagings) [PMC free article: PMC3659225] [PubMed: 23700497]
- Razdan S, Silberstein IK, Bagley DH. Ureteroscopic endoureterotomy. BJU International. 2005;95 Suppl 2:94–101. [Review] [97 refs] [PubMed: 15720342]
- Pandian SS, Hussey JK, McClinton S. Metallic ureteric stents: early experience. British Journal of Urology. 1998;82(6):791–797. [PubMed: 9883213]
- Sood G, et al. Ultrasound guided percutaneous nephrostomy for obstructive uropathy in benign and malignant diseases. International Braz J Urol. 2006;32(3):281–286. [PubMed: 16813670]
- Iwaszko MR, et al. Transureteroureterostomy revisited: long-term surgical outcomes. Journal of Urology. 2010;183(3):1055–1059. [PubMed: 20092851]
- Kehinde EO, et al. Percutaneous nephrostomies. British Journal of Urology. 1993;71(6):664–666. [PubMed: 8343890]
- Harrington KJ, et al. Palliation of obstructive nephropathy due to malignancy. British Journal of Urology. 1995;76(1):101–107. [PubMed: 7544201]
- Skat NO. Ultrasonically guided percutaneous nephrostomy. Scandinavian Journal of Urology and Nephrology. 1990;24(3):219–221. [PubMed: 1700471]
- Papatsoris AG, Buchholz N. A novel thermo-expandable ureteral metal stent for the minimally invasive management of ureteral strictures. Journal of Endourology. 2010;24(3):487–491. [PubMed: 20105033]
- Maan Z, et al. Comparison of Stent-Related Symptoms Between Conventional Double-J Stents and a New-Generation Thermoexpandable Segmental Metallic Stent: A Validated-Questionnaire-Based Study. Journal of Endourology. 2010;24(4):589–593. [PubMed: 20392160]
- Chen HC, et al. Parallel second stent placement for refractory ureteral stent malfunction in malignant ureteral obstruction. Journal of Vascular & Interventional Radiology. 2011;22(7):1012–1016. [PubMed: 21571544]
- Wenske SH. Long-term outcomes of distal ureteral reconstruction with reimplantation for benign or malignant ureteral obstruction or injury. Journal of Urology. 2012;187(4):E3–E4.
- Wenske S, Olsson CA, Benson MC. Outcomes of distal ureteral reconstruction through reimplantation with psoas hitch, Boari flap, or ureteroneocystostomy for benign or malignant ureteral obstruction or injury. Urology. 2013;82(1):231–236. [PubMed: 23642933]
- Chung HH, et al. Multicenter experience of the newly designed covered metallic ureteral stent for malignant ureteral occlusion: comparison with double j stent insertion. Cardiovascular & Interventional Radiology. 2014;37(2):463–470. [PubMed: 23925919]
- Kim JH, et al. Palliative care of malignant ureteral obstruction with polytetrafluoroethylene membrane-covered self-expandable metallic stents: initial experience. Korean Journal of Urology. 2012;53(9):625–631. [PMC free article: PMC3460005] [PubMed: 23061000]
- Del Mar TM. Percutaneous nephrostomy under fluoroscopic guidance: Technical complications. Radiologia. 1998;40(1):21–24.
- Desportes L. Neoplasic obstruction of the ureter: Drainage by percutaneous nephrostomy or double J stent. Presse Medicale. 1995;24(29):1332–1336. [PubMed: 7494843]
- El Khader K, et al. Urinary drainage by double J ureteral stents. Review of 91 cases. Annales d Urologie. 1996;30(5):235–239. [PubMed: 8975587]
- Li HZ. Clinical application of different pyelic drainage methods in the management of malignant ureteral obstruction. Journal of Interventional Radiology (China). 2012;21(9):776–779.
- Smilov N. Efficacy and safety in performing of large bore percutaneous nephrostomy under ultrasound guidance. Rentgenologiya i Radiologiya. 2009;48(3-4):186–191.
- Kouba E, Wallen EM, Pruthi RS. Management of ureteral obstruction due to advanced malignancy: optimizing therapeutic and palliative outcomes. Journal of Urology. 2008;180(2):444–450. [Review] [45 refs] [PubMed: 18550089]
- Rao MV, Polcari AJ, Turk TM. Updates on the use of ureteral stents: focus on the Resonance() stent. Medical Devices Evidence and Research. 2011;4:11–15. [PMC free article: PMC3417869] [PubMed: 22915925]
- Sountoulides P, et al. Current status of metal stents for managing malignant ureteric obstruction. BJU International. 2010;105(8):1066–1072. [Review] [PubMed: 20067458]
- Sountoulides P, Pardalidis N, Sofikitis N. Endourologic management of malignant ureteral obstruction: indications, results, and quality-of-life issues. Journal of Endourology. 2010;24(1):129–142. [PubMed: 19954354]
- Hausegger KA, Portugaller HR. Percutaneous nephrostomy and antegrade ureteral stenting: technique - indications - complications. European Radiology. 2006;16(9):2016–2030. [PubMed: 16547709]
- Liberman D, McCormack M. Renal and urologic problems: management of ureteric obstruction. Current Opinion in Supportive & Palliative Care. 2012;6(3):316–321. [Review] [PubMed: 22643703]
- Cordeiro MD. Ureteral obstruction in advanced malignancies: Prognostic factors. European Urology, Supplements. 2012.:1–e773a. Conference(var.pagings)
- Artifon EL. An interim analysis comparing EUS-Guided Anterograde Ureteral Internal Drainage Versus Percutaneous Nephrostomy in Patients With Advanced Bladder Cancer and Renal Failure: A Prospective and Randomized Trial. Gastrointestinal Endoscopy. 2012.:4. Conference(var.pagings)
- Kallidonis P. Ureteral metal stents: Ten years experience for the treatment of malignant ureteral obstruction. Journal of Endourology. 2009.:A169. Conference(var.pagings)
- Monsky W. Quality of life assessment following percutaneous nephrostomy tube versus nephroureteral stent placement for management of malignant nephroureteral obstruction. Journal of Vascular and Interventional Radiology. 2012.:3–S48. Conference(var.pagings)
- Hao P, et al. Clinical evaluation of double-pigtail sent in patients with upper urinary tract diseases: Report of 2685 cases. Journal of Endourology. 2008;22(1):65–70. [PubMed: 18177241]
Reason: not relevant to PICO
Reason: fewer than 50 participants with malignant obstruction
Reason: foreign language
Reason: expert review
Reason: meeting abstract only, insufficient information to include
Reason: duplicate study
Reason: malignant obstructions not reported
Evidence tables
Abbreviations: BCa, bladder cancer; PCN, Percutaneous nephrostomy; RC, radical cystectomy
Download PDF (395K)
5.2.3. Intractable haematuria
Review question: What specific interventions are most effective for patients with incurable bladder cancer and intractable bleeding?
Rationale
Intractable bleeding from the bladder is one of the most serious terminal complications for patients with bladder cancer because it is difficult to manage; it is frightening for the patient and their carers and almost certainly means that the patient will have to be admitted to hospital for management. Intractable bladder bleeding may occur before the patient is in a terminal phase but it may be the terminal event for bladder cancer patients. This means that they may die in hospital and certainly may lose precious hours and days that they would have rather spent at home with their family.
Severe bleeding can arise from the bladder cancer itself, radiation cystitis, cyclophosphamide induced cystitis and severe infection complicating all of these. When irrigation of the bladder through a three-way catheter fail to stop the haematuria, a life-threatening situation can develop. Blood transfusion may not keep pace with the rate of blood loss. Patients with massive uncontrollable haematuria are often elderly and already extremely frail.
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with locally advanced, metastatic bladder cancer or otherwise incurable with: Intractable bleeding | Palliative radiotherapy Palliative TURBT Urinary diversion Embolisation Palliative chemotherapy Tranexamic acid | Best supportive care Each other |
METHODS
Information sources
A literature search was performed by the information specialist (EH).
Selection of studies
The information specialist (EH) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. The full articles were then obtained for potentially relevant studies and checked against the inclusion criteria.
Data synthesis
Data from comparative studies were extracted into RevMan and risk ratios were calculated where possible. No meta-analysis was possible for this review.
RESULTS
Study quality and results
Included studies are summarised in Tables 183-185.
Evidence statements
Palliative radiotherapy
One observational study (Srinivasan et al., 1994) provided very low quality evidence about the relative effectiveness of hypofractionated (two-fraction) radiotherapy and conventional palliative radiotherapy in 41 patients selected by performance status. 59% of those receiving two-fraction radiotherapy had clearance of haematuria compared to 16% of those receiving conventional palliation (RR 3.74, 95% CI 1.25 to 11.19). One observational study of 32 patients also selected for hypofractionated radiotherapy if they had a poor performance status (Lacarriere et al., 2013). After 2 weeks of radiotherapy, 79% of patients receiving hypofractionated radiotherapy (20Gy/5 fractions/1 week) and 54% of the conventional radiotherapy (30Gy/10 fractions/2 weeks) group had complete clearance of hematuria (RR 1.47, 95% CI 0.84 to 2.55). At six months 37% and 23% in the hypofractionated and conventional radiotherapy group had no haematuria (RR 1.60, 95% CI 0.5 to 5.06).
Embolisation
Four observational studies including a total of 67 patients provided very low quality evidence for embolisation of the internal iliac arteries. Immediate control of bleeding was seen in 57/67 (85%) patients, with control rates ranging from 82% to 100% across studies. Permanent control of bleeding with mean follow-up ranging from 10 to 22 months across studies was achieved in 34/66 (51.5%) patients. The range of permanent bleeding control rates ranged from 43% to 100% across studies. After embolisation, 27% of patients required transfusion for haematuria. None of the studies reported any major treatment-related complications, except for in Jenkins et al. (1996), where one patient who did not receive prophylactic antibiotics died from septic shock 12 hours after embolisation. Ligouri et al. (2010) reported that minor complications were post-embolisation syndrome (27%), fever (11%), gluteal pain (14%), and nausea (2%).
Chemotherapy
One observational study (Mantadakis et al., 2004) provided very low quality evidence of regional intra-arterial chemotherapy (RIAC) for the symptomatic relief of patients with advanced bladder cancer who were unsuitable for surgery. Gross haematuria was present in all 32 patients prior to RIAC, which had resolved in 24/32 (75%) after treatment. There were no hemorrhagic, thrombotic or embolic complications, and no episodes of nausea or emesis. One patient developed grade three mucositis.
Table 186
GRADE evidence profile: Hypofractionated radiotherapy versus conventional palliative radiotherapy for intractable bleeding.
Table 187
GRADE evidence profile: Embolisation for intractable bleeding.
Table 188
GRADE evidence profile: Regional intra-arterial chemotherapy (RIAC) for advanced bladder cancer.
References to included studies
- El-Assmy A, Mohsen T. Internal iliac artery embolization for the control of severe bladder hemorrhage secondary to carcinoma: long-term follow-up. The scientific world journal. 2007;7:1567–1574. [PMC free article: PMC5901320] [PubMed: 17891317]
- Jenkins CNJ, McIvor J. Survival after embolization of the internal iliac arteries in ten patients with severe haematuria due to recurrent pelvic carcinoma. Clinical Radiology. 1996;51(12):865–868. [PubMed: 8972652]
- Lacarriere E, et al. The efficacy of hemostatic radiotherapy for bladder cancer-related hematuria in patients unfit for surgery. International Braz J Urol. 2013;39(6):808–816. [PubMed: 24456773]
- Liguori G, et al. Intractable haematuria: long-term results after selective embolization of the internal iliac arteries. BJU International. 2010;106(4):500–503. [PubMed: 20128777]
- Mantadakis E, et al. Symptomatic relief of patients with advanced bladder carcinoma after regional intra-arterial chemotherapy. Anticancer Research. 2003;23(6D):5143–5147. [PubMed: 14981980]
- Nabi G, et al. Therapeutic transcatheter arterial embolization in the management of intractable haemorrhage from pelvic urological malignancies: preliminary experience and long-term follow-up. BJU International. 2003;92(3):245–247. [PubMed: 12887476]
- Srinivasan V, Brown CH, Turner AG. A comparison of two radiotherapy regimens for the treatment of symptoms from advanced bladder cancer. Clinical Oncology. 1994;6(1):11–13. [PubMed: 7513538]
References to excluded studies (with reasons for exclusion)
- De Berardinis E, et al. Superselective embolization of bladder arteries in the treatment of intractable bladder haemorrhage. International Journal of Urology. 2005;12(5):503–505. [PubMed: 15948754]
- Zebic N, Weinknecht S, Kroepfl D. Radical cystectomy in patients aged > or = 75 years: an updated review of patients treated with curative and palliative intent. BJU International. 2005;95(9):1211–1214. [PubMed: 15892803]
- Malgor RD, et al. Evolution from open surgical to endovascular treatment of ureteral-iliac artery fistula. Journal of Vascular Surgery. 2012;55(4):1072–1080. [PMC free article: PMC4476467] [PubMed: 22326578]
- Sun H. Transcatheter superselective arterial embolization for the treatment of massive hemorrhage due to malignant gestational trophoblastic tumors. Journal of Interventional Radiology. 2010;19(6):447–450.
- Suvorova YV, Tarazov PG. Transcatheter embolization vs surgical ligation in the treatment of bleeding bladder neoplasms. European Journal of Cancer. 1997;33:149–149.
- Abt D, et al. Therapeutic options for intractable hematuria in advanced bladder cancer. International Journal of Urology. 2013;20(7):651–660. [PubMed: 23387805]
- Ghahestani SM, Shakhssalim N. Palliative treatment of intractable hematuria in context of advanced bladder cancer: a systematic review. Urology Journal. 2009;6(3):149–156. [Review] [40 refs] [PubMed: 19711266]
- Guven SL. Intractable Bladder Hemorrhage: Providing a Treatment Algorithm for a Complex Clinical Problem. Current Bladder Dysfunction Reports. 2011;6(4):258–264.
Reason: case study
Reason: not relevant to PICO
Reason: abstract only
Reason: expert review/not relevant to PICO
Evidence tables
Download PDF (335K)
5.2.4. Intractable pelvic pain
Review question: What specific interventions are most effective for patients with incurable bladder cancer and pelvic pain?
Rationale
Intractable pain is one of the most serious end-of-life complications for patients with bladder cancer because it is difficult to manage and it is frightening for the patient and their carers.
This review question will look primarily at medical interventions for the management of intractable pain but the location in which they are administered is also important to patients. Most patients do not want to die in hospital and would refer to die at home or in a hospice. A recent publication by the End of Life Care Intelligence Network showed that 51% of bladder cancer patients die in hospital compared with 46% for urological cancer patients as a whole.
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with incurable cancer related pelvic pain excluding pain due to bone mets | Nerve block Palliative radiotherapy Chemotherapy for bladder cancer Specialist palliative care/Pain specialist | Best supportive care, inc opioids Each other |
|
METHODS
Information sources
A literature search was performed by the information specialist (EH)
Selection of studies
The information specialist (EH) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. The full articles were then obtained for potentially relevant studies and checked against the inclusion criteria.
Data synthesis
Data from comparative studies were extracted into RevMan and risk ratios were calculated where possible. No meta-analysis was possible for this review.
RESULTS
Study quality and results
All studies identified for this evidence review were non-comparative observational studies. The evidence is summarised in Tables 189-191.
Table 189
GRADE evidence profile: Radiotherapy for cancer-related pelvic pain in patients with advanced cancer.
Table 190
GRADE evidence profile: Chemotherapy for cancer-related pelvic pain in patients with advanced cancer.
Table 191
GRADE evidence profile: Hypogastric plexus block for cancer-related pelvic pain in patients with advanced cancer.
Evidence statements
Radiotherapy
One observational study (Srinivasan et al., 1994) provided very low quality evidence about the relative effectiveness of hypofractionated (two-fraction) radiotherapy and conventional palliative radiotherapy in 41 patients selected by performance status. Pain improved in 73% of those treated with two-fraction radiotherapy compared to 37% of those treated with conventional palliation (RR 1.97, 95% CI 1.04 to 3.75). One study (58 patients) of hypofractionated radiotherapy and one study (12 patients) of short course accelerated 3D-CRT both reported a decrease in patient-reported pain after treatment, as measured on a visual analogue scale (VAS). These two studies reported an acute Grade 1-2 GI toxicity rate of 21% and an acute Grade 1-2 GU toxicity rate of 35% (Kouloulias et al., 2013; Caravatta et al., 2012). One study provided very low quality evidence for quality of life in 13 patients, reporting no statistically significant difference between baseline and post-treatment scores, although an improvement was noted in all indexes (Caravatta et al., 2012).
Chemotherapy
Very low quality evidence from one prospective nonrandomised phase II study (30 patients) of second-line gemcitabine chemotherapy in cisplatin-refractory patients, reported that VAS pain values significantly improved in the group of patients who responded to chemotherapy (Albers et al., 2002). One retrospective study of 35 patients receiving second-line gemcitabine and paclitaxel chemotherapy, reported very low quality evidence that 80% (28/35) of patients reported a decrease in VAS scores without increasing the dose of analgesics or had a decrease in analgesic consumption (Miyata et al., 2012). The most common toxicity reported in both studies was Grade 3-4 Leucopenia (36% with gemcitabine monotherapy, 14% with gemcitabine/paclitaxel). Very low quality evidence for quality of life as measured by the 10-point Spitzer scale was reported in one study (Albers et al., 2002). Mean quality of life scores for patients who did not respond to chemotherapy decreased before and after treatment (7.8 ±2.4 to 6.7 ±2.2), representing a worsening of quality of life. Quality of life scores for responders were similar before and after treatment (8.0 ±1.6 to 8.1 ±2.5).
Nerve block
Evidence of very low quality was provided by five studies reporting on the treatment of pelvic pain with a hypogastric plexus block. Two studies reported that satisfactory pain relief was achieved in 72% (133/185) of patients after one or two procedures, who all reported a VAS pain score of 8 or more out of 10 (worst possible pain) before the procedure (De Leon-Casasola et al., 1993; Plancarte et al., 1997). One study of 28 patients reported a mean pain reduction of 70% as assessed with verbal and visual analogue scales before and after treatment, although mean patient scores at baseline and follow-up were not reported (Plancarte et al., 1990). One study reported that VAS pain scores decreased from baseline at 24h, 1 week, 1 month and 2 months after treatment (p<0.05), but at three months mean scores increased and were no different from baseline (Gamal et al., 2006). Four studies (including 225 patients) provided very low quality evidence for treatment-related morbidity, with three studies reporting no intraoperative complications and one study (Gamal et al., 2006) reporting intravascular puncture (n=2, 13%) and urinary injury (n=4, 27%).
References to included studies
- Albers P, et al. Gemcitabine monotherapy as second-line treatment in cisplatin-refractory transitional cell carcinoma - prognostic factors for response and improvement of quality of life. Onkologie. 2002;25(1):47–52. [PubMed: 11893883]
- Caravatta L, et al. Short-course accelerated radiotherapy in palliative treatment of advanced pelvic malignancies: a phase I study. International Journal of Radiation Oncology, Biology, Physics. 2012;83(5):e627–e631. [PubMed: 22580117]
- Cariati M. CT-guided superior hypogastric plexus block. Journal of Computer Assisted Tomography. 2002;26(3):428–431. [PubMed: 12016374]
- De Leon-Casasola OA. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Pain. 1993;54(2):145–151. [PubMed: 8233527]
- Gamal G, Helaly M, Labib YM. Superior hypogastric block: transdiscal versus classic posterior approach in pelvic cancer pain. The.Clinical.journal of pain. 2006;22(6):544–547. [PubMed: 16788341]
- Kouloulias V, et al. Evaluation of acute toxicity and symptoms palliation in a hypofractionated weekly schedule of external radiotherapy for elderly patients with muscular invasive bladder cancer. International Braz J Urol. 2013;39(1):77–82. [PubMed: 23489500]
- Miyata Y, et al. Use of low-dose combined therapy with gemcitabine and paclitaxel for advanced urothelial cancer patients with resistance to cisplatin-containing therapy: a retrospective analysis. Cancer Chemotherapy & Pharmacology. 2012;70(3):451–459. [PMC free article: PMC3428519] [PubMed: 22864875]
- Plancarte R. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Regional Anesthesia. 1997;22(6):562–568. [PubMed: 9425974]
- Plancarte R. Superior hypogastric plexus block for pelvic cancer pain. Anesthesiology. 1990;73(2):236–239. [PubMed: 2382849]
- Srinivasan V, Brown CH, Turner AG. A comparison of two radiotherapy regimens for the treatment of symptoms from advanced bladder cancer. Clinical Oncology (Royal College of Radiologists). 1994;6(1):11–13. [PubMed: 7513538]
References to excluded studies (with reasons for exclusion)
- Mantadakis E, et al. Symptomatic relief of patients with advanced bladder carcinoma after regional intra-arterial chemotherapy. Anticancer Research. 2003;23(6D):5143–5147. Reason: outcomes not relevant to PICO . [PubMed: 14981980]
- Bosscher H. Blockade of the superior hypogastric plexus block for visceral pelvic pain. Pain Practice. 2001;1(2):162–170. Reason: expert review . [PubMed: 17129292]
- Fitzpatrick JM, et al. Treatment Decisions for Advanced Genitourinary Cancers: From Symptoms to Risk Assessment. European Urology Supplements. 2009;8(9):738–746. Reason: expert review .
- Ok JH, Meyers FJ, Evans CP. Medical and surgical palliative care of patients with urological malignancies. Journal of Urology. 2005;174(4 Pt 1):1177–1182. [Review] [48 refs] Reason: expert review . [PubMed: 16145365]
- Spagnoletti G. Palliative radiotherapy for bladder cancer: A small retrospective study. Anticancer Research. 2010.:4. Conference(var.pagings) Reason: abstract only .
- Pectasides D, et al. Combination chemotherapy with gemcitabine and ifosfamide as second-line treatment in metastatic urothelial cancer. A phase II trial conducted by the Hellenic Cooperative Oncology Group. Annals of Oncology. 2001;12(10):1417–1422. Reason: outcomes not relevant to PICO . [PubMed: 11762814]
- Sarhan TM. Male sexual dyfunction after unilateral and bilateral hypogastric plexus block for management of chronic cancer pelvic pain. European Journal of Pain Supplements. 2011;1 Conference(var.pagings) Reason: abstract only .
- Zygogianni A, et al. A weekly hypofractionated radiotherapeutic schedule for bladder carcinoma in elderly patients: local response, acute and late toxicity, dosimetric parameters and pain relief. Journal of B.U.On. 2013;18(2):407–412. Reason: population not relevant to PICO (not pelvic pain) [PubMed: 23818353]
- Patt RB. Superior hypogastric plexus block for neoplastic pelvic pain. Pain Management. 1990;3(5):259–261. Reason: review of Plancarte (1990)
- Uchibayashi T, et al. Combined treatment of radiofrequency capacitive hyperthermia for urological malignancies. Oncology Reports. 1994;1(5):937–940. Reason: intervention not relevant to PICO . [PubMed: 21607470]
- Baheti DK. Neurolytic coeliac plexus block for upper abdominal malignancies: Review of 50 cases. Pain Clinic. 1997;10(1):47–49. Reason: population/intervention not relevant to PICO .
- Bajaj P. Superior hypogastric plexus block for pelvic cancer pain. Journal of Anaesthesiology Clinical Pharmacology. 2003;19(2):161–164. Reason: majority of article is a copy of Plancarte 1990, highly unreliable paper .
Evidence tables
Download PDF (262K)
- Managing locally advanced or metastatic bladder cancer - Bladder CancerManaging locally advanced or metastatic bladder cancer - Bladder Cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...