NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Uterotonics for the prevention of postpartum haemorrhage
Review question
What is the effectiveness of uterotonics for the prevention of postpartum haemorrhage?
Introduction
Women who choose to have active management of the third stage of labour are routinely offered a uterotonic during the third stage of labour. This medicine leads to contraction of the uterus, separation of the placenta and reduces blood loss from the placental site, reducing the risk of postpartum haemorrhage (PPH). A number of different uterotonics are available including oxytocin, the oxytocin analogue carbetocin, prostaglandins and the ergot alkaloid ergometrine.
The aim of this review was to determine which uterotonic agent is the most effective and cost-effective for the prevention of postpartum haemorrhage.
Summary of the protocol
See Table 1 for a summary of the Population, Intervention, Comparison and Outcome (PICO) characteristics of this review.
Table 1
Summary of the protocol (PICO table).
For further details see the review protocol in appendix A.
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Methods specific to this review question are described in the review protocol in appendix A and the methods document (supplementary document 1).
Declarations of interest were recorded according to NICE’s conflicts of interest policy.
Effectiveness evidence
Included studies
A total of 220 randomised controlled trials (RCTs) were included in this evidence review. Most of these studies were identified from a published network meta-analysis (NMA) (n=196) (Gallos, 2018). A further 24 studies were identified by the updated literature search and included in the review.
53 studies provided evidence that was not included in the NMA and pairwise analysis was conducted for these studies (see supplement 5).
See the literature search strategy in appendix B and study selection flow chart in appendix C.
Excluded studies
Studies not included in this review are listed, and reasons for their exclusion are provided in appendix J.
Summary of included studies
There was evidence available for all the specified outcomes, but not all studies provided data for every outcome included in this evidence review, therefore a narrative summary is presented below, which describes the overall evidence, and the studies that provided evidence for specific outcomes.
Trials were predominantly in population with high risk of PPH (n=78), or a combination of high and low risk for PPH (n=73). There were 69 trials with a population of low risk for PPH. High risk for PPH was defined as women with comorbidities and pregnancy complications predisposing them to a high risk of PPH, and low risk for PPH was defined as healthy women with a singleton term pregnancy delivering vaginally.
The majority of the studies reported vaginal births (n=149). The rest reported caesarean births (n=67), both vaginal and caesarean births (n=3) and 1 study did not specify mode of birth.
Most studies included both nulliparous and multiparous women (n=125). A minority of trials included either nulliparous (n=7) or multiparous (n=1) women, and the remainder did not state the parity of participants (n=87).
Trials were predominantly conducted in women with a singleton pregnancy (n=145). Forty trials included a mixed population of women with both a singleton and multi-fetal pregnancies. One trial was conducted exclusively in women with a multi-fetal pregnancy. The remaining studies (n=35) did not explicitly state whether participants had a single or multifetal pregnancy.
Most of the trials were in a hospital setting (n=212), with only a minority in a community setting (n=8).
The majority of studies included (n=185) were 2 arm trials, directly comparing 2 different interventions, 24 studies were 3 arm trials, 9 studies were 4 arm trials and 2 were 5 arm trials.
There was evidence available for all listed interventions, apart from 2 specified injectable prostaglandins: tromethamine and sulprostone. There was evidence for all specified doses of misoprostol and oxytocin.
See the full evidence tables in appendix D (which is provided as a separate document, supplement 4) and the forest plots in appendix E.
Quality assessment of included studies
See the clinical evidence profiles in appendix D (which is provided as a separate document, supplement 4).
Clinical evidence profile for outcomes included in the network meta-analysis
NMA was used to synthesise evidence for the following outcomes (both for the whole population of women and for the subgroups of either vaginal or caesarean birth):
- PPH ≥ 1000 mL
- Additional uterotonics
- Blood transfusion
- ICU admission (severe maternal morbidity)
- Mean blood loss (mL).
Where possible, placebo was used as the reference treatment in the NMAs. In two of the outcomes for the caesarean birth subgroup, placebo was not included in the evidence network and therefore another treatment (carbetocin) was used as the reference for those two analyses.
PPH ≥ 1000ml
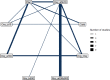
98 studies, comparing 13 interventions in 113,135 women were included in this analysis. Of these studies, 1 included women who had either vaginal or caesarean births, 71 were conducted in women who had vaginal births only, and 26 in women who had caesarean births only.
Of the 122 studies that reported this outcome, 24 studies were excluded as they reported no events in any arm for this outcome.
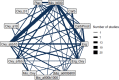
The network plot for this outcome is shown below at Figure 1, the odds ratios and log odds ratios compared to placebo in Table 2, the Forest plot at Figure 2 and the median treatment ranks in Table 3.
Table 2
Odds ratio, log odds ratio and 95% CrIs for PPH ≥ 1000 mL for all interventions compared with placebo.
In this analysis only one study included the high-dose misoprostol arm and reported no PPH ≥ 1000 mL events in that arm which led to high uncertainty in the comparison with this treatment. Therefore high-dose misoprostol has been excluded from the ranking in Table 3 as the probability of being best can be biased for highly uncertain estimates.

Figure 2
Forest plot, PPH ≥ 1000 mL full population (OR<1 favours the intervention).
Table 3
Median treatment ranks and probability of being the best treatment for all interventions for PPH ≥ 1000 mL.
Vaginal birth subgroup analysis
71 studies comparing 13 treatments in 107,322 women were included in this subgroup analysis.
Of the 91 studies that reported this outcome, 20 studies were excluded as they reported no events in any arm for this outcome.
The network plot for this outcome is shown below at Figure 3, the odds ratios compared to placebo in Table 4, the Forest plot at Figure 4 and the median treatment ranks in Table 5.
Table 4
Odds ratio, log odds ratio and 95% CrIs for PPH ≥ 1000mL for all interventions compared with placebo, vaginal birth subgroup.
In this analysis only one study included the high-dose misoprostol arm, and reported no PPH ≥ 1000 mL events in that arm which led to high uncertainty in the comparison with this treatment. Therefore high-dose misoprostol has been excluded from the ranking in Table 5 as the probability of being best can be biased for highly uncertain estimates.

Figure 4
Forest plot, PPH ≥ 1000mL vaginal birth (OR< 1 favours intervention).
Table 5
Median treatment ranks and probability of being the best treatment for all interventions for PPH ≥ 1000mL, vaginal birth subgroup.
Caesarean birth subgroup analysis
26 studies comparing 8 treatments in 5,284 women were included in this subgroup analysis.
Of the 30 studies that reported this outcome, 4 studies were excluded as they reported no events in any arm for this outcome.
The network plot for this outcome is shown below at Figure 5, the odds ratios compared to carbetocin in Table 6, the Forest plot at Figure 6 and the median treatment ranks in Table 7.
Table 6
Odds ratio, log odds ratio and 95% CrIs for PPH ≥ 1000mL for all interventions compared with carbetocin, caesarean birth subgroup.

Figure 6
Forest plot, PPH ≥ 1000mL caesarean birth (OR< 1 favours intervention, compared to carbetocin).
Table 7
Median treatment ranks and probability of being the best treatment for all interventions for PPH ≥ 1000mL, caesarean birth subgroup.
Additional uterotonics
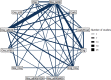
161 studies, comparing 14 interventions in 123,183 women were included in this analysis. Of these studies, 1 included women who had either vaginal or caesarean births, 109 were conducted in women who had vaginal births only, and 51 in women who had caesarean births only.
Of the 163 studies that reported this outcome, 2 studies were excluded as they reported no events in any arm for this outcome.
The network plot for this outcome is shown below at Figure 7, the odds ratios compared to placebo in Table 8, the Forest plot at Figure 8 and the median treatment ranks in Table 9.
Table 8
Odds ratio, log odds ratio and 95% CrIs for additional uterotonics for all interventions compared with placebo.

Figure 8
Forest plot, Additional uterotonics full population (OR < 1 favours intervention).
Table 9
Median treatment ranks and probability of being the best treatment for all interventions for additional uterotonics.
Vaginal birth subgroup analysis
109 studies comparing 12 treatments in 112,805 women were included in this subgroup analysis.
Of the 109 studies that reported this outcome, 0 studies were excluded as they reported no events in any arm for this outcome.
The network plot for this outcome is shown below at Figure 9, the odds ratios compared to placebo in Table 10, the Forest plot at Figure 10 and the median treatment ranks in Table 11.
Table 10
Odds ratio, log odds ratio and 95% CrIs for additional uterotonics for all interventions compared with placebo, vaginal birth subgroup.

Figure 10
Forest plot, Additional uterotonics vaginal birth (OR <1 favours intervention).
Table 11
Median treatment ranks and probability of being the best treatment for all interventions for additional uterotonics, vaginal birth subgroup.
Caesarean birth subgroup analysis
51 studies comparing 12 treatments in 10,323 women were included in this subgroup analysis.
Of the 53 studies that reported this outcome, 2 studies were excluded as they reported no events in any arm for this outcome.
The network plot for this outcome is shown below at Figure 11, the odds ratios compared to placebo in Table 12, the Forest plot at Figure 12 and the median treatment ranks in Table 13.

Figure 11
Network of evidence for additional uterotonics, caesarean birth subgroup.
Table 12
Odds ratio, log odds ratio and 95% CrIs for additional uterotonics for all interventions compared with placebo, caesarean birth subgroup.
In this analysis only one study included the ergometrine arm, and reported no additional uterotonic events in that arm which led to high uncertainty in the comparison with this treatment. Therefore, ergometrine has been excluded from the ranking in Table 13 as the probability of being best can be biased for highly uncertain estimates.

Figure 12
Forest plot, Additional uterotonics caesarean birth (OR <1 favours intervention).
Table 13
Median treatment ranks and probability of being the best treatment for all interventions for additional uterotonics, caesarean birth subgroup.
Blood transfusion
113 studies, comparing 13 interventions in 115,872 women were included in this analysis. Of these studies, 1 included women who had either vaginal or caesarean births, 80 were conducted in women who had vaginal births only, and 32 in women who had caesarean births only.
Of the 140 studies that reported this outcome, 27 studies were excluded as they reported no events in any arm for this outcome.
The network plot for this outcome is shown below at Figure 13, the odds ratios compared to placebo in Table 14, the Forest plot at Figure 14 and the median treatment ranks in Table 15.
Table 14
Odds ratio, log odds ratio and 95% CrIs for blood transfusion for all interventions compared with placebo.
In this analysis only one study included the high-dose misoprostol arm, and reported no transfusion events in that arm which led to high uncertainty in the comparison with this treatment. Therefore, high-dose misoprostol has been excluded from the ranking in Table 15 as the probability of being best can be biased for highly uncertain estimates.
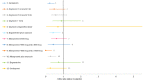
Figure 14
Forest plot, blood transfusion full population (OR< 1 favours intervention).
Table 15
Median treatment ranks and probability of being the best treatment for all interventions for blood transfusion.
Vaginal birth subgroup analysis
80 studies comparing 12 treatments in 107,850 women were included in this subgroup analysis.
Of the 99 studies that reported this outcome, 19 studies were excluded as they reported no events in any arm for this outcome.
The network plot for this outcome is shown below at Figure 15, the odds ratios compared to placebo in Table 16, the Forest plot at Figure 16 and the median treatment ranks in Table 17.
Table 16
Odds ratio, log odds ratio and 95% CrIs for blood transfusion for all interventions compared with placebo, vaginal birth subgroup.
In this analysis only one study included the high-dose misoprostol arm, and reported no transfusion events in that arm which led to high uncertainty in the comparison with this treatment. Therefore, high-dose misoprostol has been excluded from the ranking in Table 17 as the probability of being best can be biased for highly uncertain estimates.

Figure 16
Forest plot, blood transfusion vaginal birth (OR <1 favours intervention).
Table 17
Median treatment ranks and probability of being the best treatment for all interventions for blood transfusion, vaginal birth subgroup.
Caesarean birth subgroup analysis
32 studies comparing 9 treatments in 8,114 women were included in this subgroup analysis.
Of the 40 studies that reported this outcome, 8 studies were excluded as they reported no events in any arm for this outcome.
The network plot for this outcome is shown below at Figure 17, the odds ratios compared to carbetocin in Table 18, the Forest plot at Figure 18 and the median treatment ranks in Table 19.
Table 18
Odds ratio, log odds ratio and 95% CrIs for blood transfusion for all interventions compared with carbetocin, caesarean birth subgroup.
In this analysis only one study included the misoprostol >600 mcg and ≤ 800 mcg arm, and reported no transfusion events in that arm which led to high uncertainty in the comparison with this treatment. Therefore, misoprostol >600 mcg and ≤ 800 mcg has been excluded from the ranking in Table 19 as the probability of being best can be biased for highly uncertain estimates.
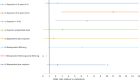
Figure 18
Forest plot, blood transfusion caesarean birth (OR <1 favours intervention compared to carbetocin).
Table 19
Median treatment ranks and probability of being the best treatment for all interventions for blood transfusion, caesarean birth subgroup.
ICU admission (morbidity)
9 studies, comparing 8 interventions in 54,377 women were included in this analysis. Of these studies, 8 were conducted in women who had vaginal births only, and 1 in women who had caesarean births only.
Of the 22 studies that reported this outcome, 13 studies were excluded as they reported no events in any arm for this outcome.
The network plot for this outcome is shown below at Figure 19, the odds ratios compared to placebo in Table 20 and the Forest plot at Figure 20.
Table 20
Odds ratio, log odds ratio and 95% CrIs for ICU admission for all interventions compared with placebo.
In this analysis all of the estimates are highly uncertain given the sparse evidence network, and the treatment ranking has not been presented as the probability of being best is likely to be biased.

Figure 20
Forest plot, ICU admission full population (OR <1 favours intervention).
The forest plot in Figure 20 does not show the point estimate or upper error bar for ergometrine plus oxytocin. This is because these values are very large, so are much further to the right on the graph than all other strategies.
Vaginal birth subgroup analysis
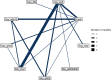
8 studies comparing 7 treatments in 54,000 women were included in this subgroup analysis.
Of the 18 studies that reported this outcome, 10 studies were excluded as they reported no events in any arm for this outcome.
The network plot for this outcome is shown below at Figure 21, the odds ratios compared to placebo in Table 21 and the Forest plot at Figure 22.

Figure 21
Network of evidence for ICU admission, vaginal birth subgroup.
Table 21
Odds ratio, log odds ratio and 95% CrIs for ICU admission for all interventions compared with placebo, vaginal birth subgroup.
In this analysis all of the estimates are highly uncertain given the sparse evidence network, and the treatment ranking has not been presented as the probability of being best is likely to be biased.
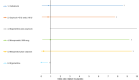
Figure 22
Forest plot, ICU admission vaginal birth (OR <1 favours intervention).
The forest plot in Figure 22 does not show the point estimate or upper error bar for ergometrine plus oxytocin. This is because these values are very large, so are much further to the right on the graph than all other strategies.
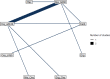
Caesarean birth subgroup analysis
Only 1 study comparing 2 treatments was identified for this outcome in the caesarean birth subgroup, therefore an NMA could not be conducted. These results were analysed using pairwise analysis, which can be found in supplement 5.
Mean blood loss (ml)
156 studies, comparing 14 interventions in 85,514 women were included in this analysis. Of these studies, 1 included women who had either vaginal or caesarean births, 109 were conducted in women who had vaginal births only, and 46 in women who had caesarean births only.
The network plot for this outcome is shown below at Figure 23, the odds ratios compared to placebo in Table 22, the Forest plot at Figure 24 and the median treatment ranks in Table 23.
Table 22
blood loss ratio and 95% CrI for mean blood loss for all interventions compared with placebo.

Figure 24
Forest plot, mean blood loss full population (OR< 1 favours intervention).
Table 23
Median treatment ranks and probability of being the best treatment for all interventions for mean blood loss.
Vaginal birth subgroup analysis
109 studies comparing 13 treatments in 76,400 women were included in this subgroup analysis.
The network plot for this outcome is shown below at Figure 25, the odds ratios compared to placebo in Table 24, the Forest plot at Figure 26 and the median treatment ranks in Table 25.
Table 24
blood loss ratio and 95% CrI for mean blood loss for all interventions compared with placebo, vaginal birth subgroup.
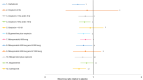
Figure 26
Forest plot, mean blood loss vaginal birth (OR < 1 favours intervention).
Table 25
Median treatment ranks and probability of being the best treatment for all interventions for mean blood loss, vaginal birth subgroup.
Caesarean birth subgroup analysis
46 studies comparing 12 treatments in 8,585 women were included in this subgroup analysis.
The network plot for this outcome is shown below at Figure 27, the odds ratios compared to placebo in Table 26, the Forest plot at Figure 28 and the median treatment ranks in Table 27.
Table 26
blood loss ratio and 95% CrI for mean blood loss for all interventions compared with placebo, caesarean birth subgroup.

Figure 28
Forest plot, mean blood loss caesarean birth (OR < 1 favours intervention).
Table 27
Median treatment ranks and probability of being the best treatment for all interventions for mean blood loss, caesarean birth subgroup.
Economic evidence
Included studies
Two economic studies were identified which were relevant to this question (Gallos 2019 and Matthijsse 2022).
See the literature search strategy in Appendix B and economic study selection flow chart in Appendix G.
Excluded studies
Economic studies not included in this review are listed, and reasons for their exclusion are provided in Appendix J.
Summary of included economic evidence
See Table 28 for the economic evidence profile of the included studies.
Table 28
Economic evidence profile of a systematic review of economic evaluations of uterotonics for the prevention of postpartum haemorrhage.
Economic model
A de-novo economic model was developed to answer this question, based on the outputs from the updated NMAs on PPH ≥1000 mL, additional uterotonics, ICU admission, and blood transfusions. The model utilised the decision tree structure as shown in Figure 29, with the time horizon only considering the immediate costs and outcomes in the third stage of labour. The model compares all uterotonics included in the NMAs (where data allows), and due to a lack of utility data in this area, costs are presented alongside outcomes such as number of PPH≥1000 mL events avoided rather than as cost per QALY gained.
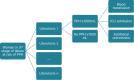
Figure 29
Model schematic.
Costs included in the model were; prophylactic drug costs, drug administration costs, treatment-related adverse event costs, cost of additional uterotonics, cost of blood transfusion, and cost of ICU admission (as a scenario analysis). In the base-case, ICU admission and the costs associated with this were excluded due to missing data in the NMA.
Results were generated separately for the full population and the subgroups of vaginal birth and caesarean birth, as agreed by the committee. Efficacy results for each population group were informed by the corresponding NMA.
Deterministic results were presented alongside a probabilistic sensitivity analysis (PSA) which involved repeated Monte Carlo simulation of model inputs from their corresponding probability distributions. This was done in order to capture the inherent uncertainty in the model inputs. In each simulation the total cost, and total number of outcomes was calculated for each uterotonic. These individual simulation values were then aggregated to determine the average total cost and total number of outcomes, to evaluate the cost per event avoided. The probabilistic results were broadly similar to the deterministic results, suggesting that the cost-effectiveness results are fairly stable to the parameter uncertainty.
Scenario analyses were conducted on inclusion of ICU admissions in the model, exclusion of adverse events, and route of administration for carbetocin, to determine the impact of these events in the economic results. These scenarios were selected as there were data gaps and therefore uncertainty in the data informing these aspects of the economic analysis.
For full details of the economic model and results are available in appendix I.
Unit costs
The drug costs used in the economic model are detailed in the table below. Where combinations (for example, misoprostol plus oxytocin) or different doses than those listed (for example, misoprostol >800 mcg and ≤ 1000 mcg) have been modelled, the costs have been calculated using the values shown in the table. Details of these calculations are available in appendix I, Table 34.
| Resource | Unit costs | Source |
|---|---|---|
| Carbetocin 100mcg | £17.64 (per dose) | British National Formulary - January 2023 |
| Oxytocin 5IU | £0.80 (per dose) | British National Formulary - January 2023 |
| Oxytocin 10IU | £0.91 (per dose) | British National Formulary - January 2023 |
| Ergometrine 500mcg | £1.50 (per dose) | British National Formulary - January 2023 |
| Misoprostol 200mcg | £0.17 (per dose) | British National Formulary - January 2023 |
| Carboprost 250mcg | £18.20 (per dose) | British National Formulary - January 2023 |
| Syntometrine | £1.57 (per dose) | British National Formulary - January 2023 |
The committee's discussion and interpretation of the evidence
The outcomes that matter most
The committee chose postpartum haemorrhage greater than or equal to 1000 mL as the critical outcome for this review. They agreed that this outcome directly informed the effectiveness of uterotonics to prevent postpartum haemorrhage. In addition, they agreed that this outcome indicated major haemorrhage which would impact the woman’s birth experience, ability to bond with the baby, and could have a psychological impact on both her and her partner. They also agreed on important outcomes for the review as the need for additional uterotonics, intensive care admissions, number of blood transfusions, and mean blood loss volume. They agreed that these outcomes would provide further information to help understand which uterotonic would be the most effective to prevent postpartum haemorrhage, as they would demonstrate a reduction in other negative outcomes or need for further interventions. The committee discussed maternal mortality and agreed on the high importance of this outcome. However, they agreed that although postpartum haemorrhage accounts for many of maternal deaths, maternal mortality is still a very rare outcome and many studies would be underpowered to report this outcome with precision. Therefore the committee agreed that looking at postpartum haemorrhage as an outcome would be the best way of approaching this review question.
The quality of the evidence
The trials included for this evidence review were individually assessed using the Cochrane risk of bias tool, and the summarised quality of the evidence for each of the NMAs is presented in supplement 4. The main area where trials were at risk of bias was due to not blinding of assessors. There were also some concerns around allocation concealment, and unclear bias on selective reporting due to no protocol being available to review.
The data presented using pairwise analysis were assessed using GRADE. The majority of the comparisons were assessed as low to very low. The concerns were mainly due to imprecision and also some concerns around risk of bias.
The inconsistency checks highlighted moderate heterogeneity but little evidence of inconsistency (see appendix N for more information).
Benefits and harms
The committee discussed the network meta-analysis evidence on the use of a variety of uterotonics for the prevention of postpartum haemorrhage. They considered the evidence for the whole population for the critical outcome of PPH of 1000 mL or more and noted that the evidence indicated that, based on the odds ratios, all active treatments were better than placebo, although for 2 treatments the confidence intervals did cross the line of no effect. The committee therefore focussed their discussion on which of the treatments was most effective and noted that there were differences in the most effective uterotonic across the outcomes depending on mode of birth, and therefore agreed that they would consider the evidence broken down by modes of birth (vaginal or caesarean).
The committee also discussed that the evidence for ICU admission (as an indicator of maternal morbidity) was based on evidence from only 9 studies, the networks were sparsely populated, and in the case of the caesarean birth sub-group non-existent, so they agreed that the results from this outcome would be less influential in helping them make decisions.
The committee discussed the method of administration of the uterotonics and noted that both oxytocin and carbetocin could be administered by intravenous or intramuscular injection. They noted that the evidence for both oxytocin and carbetocin included both these routes of administration.
The committee discussed that in addition to the clinical effectiveness of the drugs it was also important to consider the route of administration, licensed status, cost effectiveness, side effects and heat stability.
The committee first discussed the evidence for the caesarean birth subgroup. They discussed that across the outcomes of postpartum haemorrhage of 1000 mL or more, need for additional uterotonics and blood transfusion, the evidence showed that misoprostol >600 mcg to ≤800 mcg and carbetocin were the best ranked drugs, although there was some uncertainty due to large credible intervals. For the outcome of mean blood loss, misoprostol plus oxytocin, or carbetocin were the best ranked drugs. As outlined above, the committee agreed that the evidence for intensive care admission was limited and they could not use it to guide their recommendations. The committee therefore discussed that misoprostol or carbetocin could be options for women who had had a caesarean birth but agreed that in theatre it would be easier to give intravenous carbetocin rather than misoprostol which has to be given orally or rectally, and also that misoprostol is not approved for this indication whereas carbetocin is approved. They also discussed the importance of side effects and noted that misoprostol often causes nausea and vomiting, diarrhoea and abdominal pain which would be extremely unpleasant for the woman, and may be difficult for woman being sutured and recovering from a caesarean birth. The committee also discussed the impact of these side-effects on the birth experience of the woman and her partner and agreed that although they might be short term, the postpartum period is a crucial period for bonding with the baby and it was important to ensure the best experience for the woman and her partner. The committee discussed that carbetocin was heat stable and did not require refrigeration, although this was unlikely to cause a problem in theatres where fridges for drug storage were likely to be available.
The committee then reviewed the cost-effectiveness data for the caesarean birth population (see more detailed discussion below) and agreed that due to its clinical and cost effectiveness they would recommend intravenous carbetocin to prevent PPH in those women having a caesarean birth.
The committee then looked at the evidence for the vaginal birth subgroup and agreed that the results for this group were more varied. For the critical outcome of PPH of 1000 mL or more there was evidence that some doses of oxytocin, ergometrine, ergometrine plus oxytocin, carbetocin, some doses of misoprostol or misoprostol plus oxytocin were all effective. Similarly, a number of these treatments and carboprost seemed to demonstrate similar efficacy for the outcomes of additional uterotonics, blood transfusion and mean blood loss. The committee agreed not to recommend misoprostol because, as for caesarean birth, they were aware that misoprostol would have unpleasant side effects of nausea and vomiting, diarrhoea and abdominal pain and this would impact the woman’s birth experience, and that it was not approved for this indication. The committee agreed that oxytocin or oxytocin plus ergometrine were already routinely used for prophylaxis of PPH, could be given intramuscularly in home births or midwife-led settings and that either of these options could be recommended. They also discussed the place of carbetocin but used the health economic analysis to inform their decision to not recommend carbetocin for use after vaginal birth as it was not cost-effective (see more detailed discussion below).
The committee discussed the evidence for oxytocin, and for oxytocin plus ergometrine, which both showed similar effectiveness for the outcomes of the review, although oxytocin plus ergometrine was marginally more effective with tighter credible intervals for the critical outcome of PPH of 1000 mL or more, and noted that oxytocin >5 units and ≤ 10 units and oxytocin plus ergometrine were cost-effective (see detailed discussion below). They agreed to offer women the option of either of these 2 drugs. The committee discussed that the side effect profiles differ between the two drugs, and that oxytocin plus ergometrine could be associated with more side effects (for example, nausea and vomiting) than oxytocin alone, and was contraindicated in women with severe hypertension, cardiac, hepatic or renal disease. Nevertheless, the committee agreed that it was important to give women the option for either drug given the potential increased effectiveness of oxytocin plus ergometrine, but that there needed to be careful consideration of the benefits and harms to allow women to make an informed decision. Based on their knowledge and experience the committee agreed that women receiving oxytocin plus ergometrine should be offered antiemetics as well.
The committee discussed that the evidence was not stratified by risk of postpartum haemorrhage and that the population in the evidence included a mixed population of high and low risk. They discussed that the recommendations would apply to a general mixed population but the committee agreed that due to the marginal greater effectiveness of oxytocin plus ergometrine they would advise this option in women who had been identified as at a higher risk of PPH.
Cost effectiveness and resource use
A health economic model was developed for this review question, comparing uterotonics for preventing postpartum haemorrhage. The committee also considered the two published studies identified for this review question (Gallos 2019 and Matthijsse 2022), but ultimately based their recommendations on the updated NMA and new economic model as that included the more recent clinical evidence including 2 large carbetocin trials, and also differentiated treatments by dose. The model was run separately for vaginal birth and caesarean birth since outcomes in the NMA were generated for each of these populations and the committee thought that there would be important differences between these groups and that recommendations may need to be considered separately.
The committee did not use the results of the full population analysis to make recommendations as they felt it was more appropriate to consider the mode-of-birth subgroups separately given the differences between them.
The results from the economic model included three outcomes alongside costs; PPH ≥ 1000 mL, need for additional uterotonics, and need for blood transfusions. The committee reviewed all results generated in the model, but primarily based their decisions on the PPH ≥ 1000 mL outcome as this was designated as the critical outcome for this review.
There was evidence in the caesarean birth subgroup suggesting carbetocin to be the most cost-effective option of the uterotonics the committee were considering. Carbetocin was more costly but more effective than oxytocin >1 iu and ≤ 5 iu, and dominant over other oxytocin doses and oxytocin plus ergometrine. Using the conversion method described in a published HTA (Gallos 2019), carbetocin would be considered cost effective compared with oxytocin if a person would be willing to trade 17 days in full health to avoid having a PPH ≥ 1000 mL. The committee agreed that this was a reasonable trade off and recommended that carbetocin be offered for women who have had a caesarean birth.
In the vaginal birth subgroup the committee agreed that oxytocin >5 iu and ≤ 10 iu was likely to be the most cost-effective option, and oxytocin plus ergometrine was more costly and more effective but would be considered cost-effective compared to oxytocin if a person was willing to trade 91 days of full health to avoid having a PPH ≥ 1000 mL. However, there was missing data in the information from the HTA informing the adverse events in the model and a scenario analysis was undertaken in which side-effects were excluded. The committee considered this scenario as they believed that some of the key side effects of treatments in the comparison (nausea and vomiting) could be mitigated by offering antiemetics. Under this scenario oxytocin plus ergometrine became the most likely cost-effective option, being least costly and most effective. Carbetocin was more costly and less effective than oxytocin plus ergometrine in the base case model and in the scenario where adverse events were included. In the scenario where the cost of intramuscular injection was used for carbetocin administration carbetocin was less costly and less effective than ergometrine plus oxytocin, but more costly and more effective than oxytocin >5 iu and ≤ 10 iu, however when carbetocin was assumed to be administered by intramuscular injection and adverse events were excluded carbetocin was still more costly and less effective than oxytocin plus ergometrine. The committee agreed that the cost-effectiveness evidence was not strong enough to recommend carbetocin for the prevention of postpartum haemorrhage following vaginal birth. Based on the evidence, the committee recommended a choice between oxytocin or oxytocin plus ergometrine and listed factors that should be discussed when making this decision. The committee also recommended that antiemetics are offered alongside oxytocin plus ergometrine due to the higher likelihood of nausea and vomiting. Offering antiemetics is not anticipated to have a substantial resource impact as these drugs are low cost and are expected to reduce downstream costs in terms of managing nausea and vomiting.
Other factors the committee took into account
The committee noted that the use of oxytocin for the prevention of postpartum haemorrhage was within the licensed indications and although the IM route is not strictly in line with the marketing authorisation, they recognised that this a well-established practice and licensed in several other countries.
Recommendations supported by this evidence review
This evidence review supports recommendations 1.10.9 to 1.10.11 and 1.10.13.
References
Abdel-Aleem 2010
Abdel-Aleem H, Singata M, Abdel-Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. International Journal of Gynecology & Obstetrics 2010;111(1):32–6. [PubMed: 20599196]Acharya 2001
Acharya G, Al-Sammarai MT, Patel N, Al-Habib A, Kiserud T. A randomized, controlled trial comparing effect of oral misoprostol and intravenous syntocinon on intra-operative blood loss during cesarean section. Acta Obstetricia et Gynecologica Scandinavica 2001;80(3):245–50. [PubMed: 11207490]Adanikin 2012
Adanikin AI, Orji EO, Adanikin PO, Olaniyan O. Comparative study of rectal misoprostol to oxytocin infusion in preventing postpartum haemorrhage post-caesarean section. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S825.- •Adanikin AI, Orji EO, Fasubaa OB, Onwudiegwu U, Ijarotimi OA, Olaniyan O. The effect of post-cesarean rectal misoprostol on intestinal motility. International Journal of Gynecology and Obstetrics 2012;119(2):159–62. [PubMed: 22925817]
- •Orji EO, Adanikin AI. Prospective randomised double blind study on the effect of post-caesarean rectal misoprostol on intestinal motility. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S446–S447. [PubMed: 22925817]
- •Orji EO, Adanikin AO. The effect of post-caesarean rectal misoprostol on intestinal motility. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):21. [PubMed: 22925817]
Afolabi 2010
Afolabi EO, Kuti O, Orji EO, Ogunniyi SO. Oral misoprostol versus intramuscular oxytocin in the active management of the third stage of labour. Singapore Medical Journal 2010;51(3):207–11. [PubMed: 20428741]Ahmed 2014
Ahmed WAS, Ibrahim ZM, Mostafa I, Kishk EA, Elbahie MA. Safety and efficacy of carbetocin in hypertensive pregnant women undergoing cesarean delivery. Journal of Maternal-Fetal & Neonatal Medicine 2014;27(Suppl 1):49.Al- Sawaf 2013
Al-Sawaf A, El-Mazny A, Shohayeb A. A randomised controlled trial of sublingual misoprostol and intramuscular oxytocin for prevention of postpartum haemorrhage. Journal of Obstetrics and Gynaecology 2013;33(3):277–9 [PubMed: 23550857]Al-Zubaidi 2022
Al Zubaidi Shaymaa and Alhaidari Taghreed. Heat stable carbetocin vs. oxytocin for the prevention of post-partum hemorrhage in emergency caesarean delivery: a randomized controlled trial. Journal of perinatal medicine 2022 50(2): 150–156 [PubMed: 34535047]Amant 1999
Amant F, Spitz B, Timmerman D, Corresmans A, Assche FA. Misoprostol compared with methylergometrine for the prevention of postpartum haemorrhage: a double-blind randomised trial. British Journal of Obstetrics and Gynaecology 1999;106:1066–70. [PubMed: 10519433]Amin 2014
Amin N. Prophylactic use of misoprostol in management of third stage of labour and prevention of atonic uterus. Journal of Postgraduate Medical Institute 2014;28(2):196–200.Amornpetchakul 2018
Amornpetchakul Paweena, Lertbunnaphong Tripop, Boriboonhiransarn Dittakarn et al . Intravenous carbetocin versus intravenous oxytocin for preventing atonic postpartum hemorrhage after normal vaginal delivery in high-risk singleton pregnancies: a triple-blind randomized controlled trial. Archives of gynecology and obstetrics 2018; 298(2): 319–327 [PubMed: 29916110]Anupama 2021
Anupama Sublingual Misoprostol for the Prevention of Postpartum Haemorrhage - A Randomised Control Trial. European Journal of Molecular and Clinical Medicine 2021; 8(4): 2218–2221Askar 2011
Askar AA, Ismail MT, El-Ezz AA, Rabie NH. Carbetocin versus syntometrine in the management of third stage of labor following vaginal delivery. Archives of Gynecology and Obstetrics 2011;284(6):1359–65. [PubMed: 21336835]Attilakos 2008
Attilakos G, Psaroudakis D, Ash J, Buchanan, Winter C, Donald F, et al Can a new oxytocin analogue reduce the need for additional oxytocics after caesarean section? The results of a double-blind randomised trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa51.- •Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double-blind randomised trial. BJOG: an international journal of obstetrics and gynaecology 2010;117(8):929–36. [PubMed: 20482535]
- •Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al The haemodynamic effects of oxytocin and carbetocin following caesarean section: the results of a double-blind randomised study. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):140–1. [PubMed: 20482535]
- •Attilakos G, Psaroudakis, Ash J, Buchanen R, Winter C, Draycott T. Low recruitment rate for a drug trial in obstetrics: an effect of the publicity following the TGN1412 clinical trial at the PAREXEL Research Unit in Northwick Park Hospital? [abstract]. 31st British International Congress of Obstetrics and Gynaecology; 2007 July 4–6; London, UK. 2007:110.
Atukunda 2014
Atukunda EC, Siedner MJ, Obua C, Mugyenyi GR, Twagirumukiza M, Agaba AG. Sublingual misoprostol versus intramuscular oxytocin for prevention of post-partum haemorrhage in Uganda: a randomised, controlled, non-inferiority trial. Lancet 2014;384(Suppl 1):S3. [PMC free article: PMC4219663] [PubMed: 25369200]- •Atukunda EC, Siedner MJ, Obua C, Mugyenyi GR, Twagirumukiza M, Agaba AG. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in Uganda: a double-blind randomized non-inferiority trial. PLoS Medicine 2014;11(11):e1001752. [PMC free article: PMC4219663] [PubMed: 25369200]
Badejoko 2012
Badejoko OO, Ijarotimi AO, Awowole IO, Loto OM, Badejoko BO, Olaiya DS, et al Adjunctive rectal misoprostol versus oxytocin infusion for prevention of postpartum hemorrhage in women at risk: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2012;38(11):1294–301 [PubMed: 22612662]Bagheri 2022
Bagheri Fatemeh Zahra, Azadehrah Mahboobeh, Shabankhani Bizhan et al Rectal vs. sublingual misoprostol in cesarean section: Three-arm, randomized clinical trial. Caspian journal of internal medicine 2022; 13(1): 84–89 [PMC free article: PMC8797813] [PubMed: 35178212]Balki 2008
Balki M, Dhumne S, Kasodekar S, Kingdom J, Windrim R, Carvalho JC. Oxytocin-ergometrine co-administration does not reduce blood loss at caesarean delivery for labour arrest. BJOG: an international journal of obstetrics and gynaecology 2008;115(5):579–84. [PubMed: 18333937]Balki 2021
Balki Mrinalini, Downey Kristi, Walker Andrew et al Prophylactic Administration of Uterotonics to Prevent Postpartum Hemorrhage in Women Undergoing Cesarean Delivery for Arrest of Labor: A Randomized Controlled Trial. Obstetrics and gynecology 2021; 137(3): 505–513 [PubMed: 33543897]Bamigboye 1998
Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum haemorrhage: a placebo controlled trial. Proceedings of the 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:49–52. [PubMed: 9790395]Bamigboye 1998
Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum hemorrhage: a placebo-controlled trial. American Journal of Obstetrics and Gynecology 1998;179(4):1043–6. [PubMed: 9790395]Bamigboye 1998
Bamigboye AA, Merrell DA, Hofmeyr GJ, Mitchell R. Randomized comparison of rectal misoprostol with syntometrine for management of third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 1998;77:178–81. [PubMed: 9512323]Barton 1996
Barton SR, Jackson A. The safety and efficiency of carbetocin to control uterine bleeding following caesarean section. Prenatal and Neonatal Medicine 1996;1(Suppl 1):185.Baskett 2005
Baskett TF, Persad V, Clough H, Young D. Prophylactic use of misoprostol in the third stage of labor [abstract]. Obstetrics & Gynecology 2005;105(4 Suppl):39S.- •Baskett TF, Persad VL, Clough HJ, Young DC. Misoprostol versus oxytocin for the reduction of postpartum blood loss. International Journal of Gynecology & Obstetrics 2007;97(1):2–5. [PubMed: 17321529]
- •Chandra S, Persad V, Young D, Baskett T. A preliminary study of cutaneous blood flow associated with postpartum use of oral misoprostol. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(12):1073–6. [PubMed: 15607043]
Begley 1990
Begley CM. Comparative studies in the third stage of labour [MSc thesis]. Dublin: Trinity College, University of Dublin, 1990.- •Begley CM. A comparison of 'active' and 'physiological' management of the third stage of labour. Midwifery 1990;6:3–17. [PubMed: 2182978]
- •Begley CM. The effect of ergometrine on breast feeding. Midwifery 1990;6:60–72. [PubMed: 2195299]
Bellad 2009
Bellad M, Ganachari TDM, Mallapur M. Sublingual (SL) powdered misoprostol (400 mcg) vs IM oxytocin (10 IU) for prevention of postpartum blood loss - a randomized controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S124–5.- •Bellad MB, Tara D, Ganachari MS, Mallapur MD, Goudar SS, Kodkany BS, et al Prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin: a double-blind randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2012;119(8):975–86. [PubMed: 22703421]
Benchimol 2001
Benchimol M, Gondry J, Mention JE, Gagneur O, Boulanger JC. Role of misoprostol in the delivery outcome. [French] [Place du misoprostol dans la direction de la delivrance.]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2001;30(6):576–83. [PubMed: 11883025]Bhullar 2001
Bhullar A, Carlan SJ, Hamm J, Lamberty N, White L, Richichi K. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstetrics & Gynecology 2004;104(6):1282–8. [PubMed: 15572491]Borruto 2009
Borruto F, Treisser A, Comparetto C. Utilization of carbetocin for prevention of postpartum hemorrhage after cesarean section: a randomized clinical trial. Archives of Gynecology and Obstetrics 2009;280(5):707–12. [PubMed: 19229549]Boucher 1998
Boucher M, Horbay GL, Griffin P, Deschamps Y, Desjardins C, Schulz M, et al Double-blind, randomized comparison of the effect of carbetocin and oxytocin on intraoperative blood loss and uterine tone of patients undergoing cesarean section. Journal of Perinatology 1998;18(3):202–7. [PubMed: 9659650]Boucher 2001
Boucher M, Nimrod C, Tawagi G. Carbetocin IM injection vs oxytocin IV infusion for prevention of postpartum hemorrhage in women at risk following vaginal delivery. American Journal of Obstetrics and Gynecology 2001;185(6 Pt 2):Abstract no: 494.- •Boucher M, Nimrod CA, Tawagi GF, Meeker TA, Rennicks White RE, Varin J. Comparison of carbetocin and oxytocin for the prevention of postpartum hemorrhage following vaginal delivery:a double-blind randomized trial. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(5):481–8. [PubMed: 15151735]
Bugalho 2001
Bugalho A, Daniel A, Foundes A, Cunha M. Misoprostol for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2001;73:1–6. [PubMed: 11336714]Butwick 2009
Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. A study of the minimum effective dose of oxytocin in patients undergoing elective cesarean delivery. American Society of Anaesthesiologists Annual Meeting; 2009 Oct 17–21; New Orleans, USA. 2009.- •Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. Minimum effective bolus dose of oxytocin during elective caesarean delivery. British Journal of Anaesthesia 2010;104(3):338–43. [PubMed: 20150347]
Caliskan 2002
Caliskan E, Meydanli M, Dilbaz B, Aykan B, Sonmezer M, Haberal A. Is rectal misoprostol really effective in the treatment of third stage of labor? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2002;187:1038–45. [PubMed: 12389002]Caliskan 2003
Caliskan E, Dilbaz B, Meydanli MM, Ozturk N, Narin MA, Haberal A. Oral misoprostol for the third stage of labor: a randomized controlled trial. Obstetrics & Gynecology 2003;101(5 Pt 1):921–8. [PubMed: 12738151]Carbonell 2009
Carbonell i Esteve JL, Hernandez JMR, Piloto M, Setien SA, Texido CS, Tomasi G, et al Active management of the third phase of labour plus 400 mug of sublingual misoprostol and 200 mug of rectal misoprostol versus active management only in the prevention of post-partum haemorrhage. A randomised clinical trial [Manejo activo de la tercera fase del parto mas 400 mug de misoprostol sublingual y 200 mug de misoprostol rectal frente a manejo activo solo en la prevencion de la hemorragia posparto. Ensayo clinico aleatorizado]. Progresos de Obstetricia y Ginecologia 2009;52(10):543–51.Cayan 2010
Cayan F, Doruk A, Sungur MA, Dilek S. Comparison of the different dosages of rectal misoprostol on I=intestinal motility and pain score in high risk cesarean delivery. Turkiye Klinikleri Journal of Medical Sciences 2010;30(4):1154–9.Chaudhuri 2010
Chaudhuri P, Banerjee GB, Mandal A. Rectally administered misoprostol versus intravenous oxytocin infusion during cesarean delivery to reduce intraoperative and postoperative blood loss. International Journal of Gynecology & Obstetrics 2010;109(1):25–9. [PubMed: 20070961]Chaudhuri 2012
Chaudhuri P, Biswas J, Mandal A. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in low-risk women. International Journal of Gynecology and Obstetrics 2012;116(2):138–42. [PubMed: 22100204]Chaudhuri 2015
Chaudhuri P, Majumdar A. Sublingual misoprostol as an adjunct to oxytocin during cesarean delivery in women at risk of postpartum hemorrhage. International Journal of Gynaecology & Obstetrics 2015;128:48–52. [PubMed: 25277789]Chhabra 2008
Chhabra S, Tickoo C. Low-dose sublingual misoprostol versus methylergometrine for active management of the third stage of labor. Journal of Obstetrics and Gynaecology Research 2008;34(5):820–3. [PubMed: 18834340]Choy 2002
Choy CMY, Lau WC, Tam WH, Yuen PM. A randomised controlled trial of intramuscular syntometrine and intravenous oxytocin in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2002;109:173–7. [PubMed: 11905429]Cook 1999
Cook CM, Spurrett B, Murray H. A randomized clinical trial comparing oral misoprostol with synthetic oxytocin or syntometrine in the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1999;39(4):414–9. [PubMed: 10687755]Dansereau 1996
Dansereau J. Comparison of carbetocin vs. oxytocin in prevention of uterine atony post cesarean section. Prenatal and Neonatal Medicine 1996;1(Suppl 1):80.- •Dansereau J, Gambling D, Joshi A, Helewa M, Doran T, Lange I, et al Double-blind comparison of carbetocin vs oxytocin in preventing uterine atony post Cesarean section. International Journal of Gynecology & Obstetrics 1994;46 Suppl:77.
- •Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, et al Double blind comparison of carbetocin versus oxytocin in prevention of uterine atony after cesarean section. American Journal of Obstetrics and Gynecology 1999;18(3 Pt 1):670–6. [PubMed: 10076146]
- •Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, et al Double-blind comparison of carbetocin vs oxytocin in preventing uterine atony post Cesarean section. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;69:37. [PubMed: 10076146]
- •Gambling D, Dansereau J, Schulz M, Horbay GLA, Waasenaar W. Double-blind, randomized comparison of a single dose of carbetocin vs 8 hours oxytocin infusion after cesarean delivery: safety data. A Canadian multi-center trial [abstract]. International Journal of Obstetric Anesthesia 1994;3:113–4.
- •Gambling DR, Dansereau J, Wassenaar W, Schulz M, Horbay GLA. Double-blind randomized comparison of a single dose of carbetocin versus 8 hours oxytocin infusion after cesarean delivery: safety data. Anesthesia & Analgesia 1994;78 Suppl:S127.
Dasuki 2002
Dasuki D, Emilia O, Harini S. Randomized clinical trial: the effectiveness of oral misoprostol versus oxytocin in prevention of postpartum hemorrhage [abstract]. Journal of Obstetrics and Gynaecology Research 2002;28(1):46.Groot 1996
Groot ANJA, Roosmalen J, Dongen PWJ, Borm GF. A placebo-controlled trial of oral ergometrine to reduce postpartum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 1996;75:464–8. [PubMed: 8677772]Derman 2006
•Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial. Lancet 2006;368(9543):1248–53. [PubMed: 17027730]- •Geller SE, Goudar SS, Adams MG, Naik VA, Patel A, Bellad MB, et al Factors associated with acute postpartum hemorrhage in low-risk women delivering in rural India. International Journal of Gynecology & Obstetrics 2008;101(1):94–9. [PMC free article: PMC3711742] [PubMed: 18291401]
- •Geller SE, Patel A, Niak VA, Goudar SS, Edlavitch SA, Kodkany BS, et al Conducting international collaborative research in developing nations. International Journal of Gynecology & Obstetrics 2004;87(3):267–71. [PubMed: 15548406]
- •Goudar SS, Chakraborty H, Edlavitch SA, Naik VA, Bellad MB, Patted SS, et al Variation in the postpartum hemorrhage rate in a clinical trial of oral misoprostol. Journal of Maternal-Fetal & Neonatal Medicine 2008;21(8):559–64. [PubMed: 18609354]
- •Kodkany BS, Derman RJ, Goudar SS, Geller SE, Edlavitch SA, Naik VA, et al Initiating a novel therapy in preventing postpartum hemorrhage in rural India: a joint collaboration between the United States and India. International Journal of Fertility & Womens Medicine 2004;49(2):91–6. [PubMed: 15188836]
- •Kodkany BS, Goudar SS, Derman RJ. The efficacy of oral misoprostol in preventing postpartum hemorrhage in a community setting: a randomized double-blind placebo-controlled trial. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S141–S142. [PubMed: 29644698]
- •NCT00097123. RCT of misoprostol for postpartum hemorrhage in India. clinicaltrials.gov/ct2/show/NCT00097123 (first received 18 November 2004).
- •Patted SS, Goudar SS, Naik VA, Bellad MB, Edlavitch SA, Kodkany BS, et al Side effects of oral misoprostol for the prevention of postpartum hemorrhage: results of a community-based randomised controlled trial in rural India. Journal of Maternal-Fetal & Neonatal Medicine 2009;22(1):24–8. [PubMed: 19089777]
Dhananjaya 2014
Dhananjaya BS, Charishma S. Comparative study of efficacy and safety of intramuscular oxytocin with intramuscular methylergometrine in the active management of third stage of labour. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5(3):734–9.Docherty 1981
Docherty PW, Hooper M. Choice of an oxytocic agent for routine use at delivery. Journal of Obstetrics and Gynaecology 1981;2:60.Eftekhari 2009
Eftekhari N, Doroodian M, Lashkarizadeh R. The effect of sublingual misoprostol versus intravenous oxytocin in reducing bleeding after caesarean section. Journal of Obstetrics and Gynaecology 2009;29(7):633–6. [PubMed: 19757270]Behery 2015
Behery MM, Sayed GA, Hameed AA, Soliman BS, Abdelsalam WA, Bahaa A. Carbetocin versus oxytocin for prevention of postpartum hemorrhage in obese nulliparous women undergoing emergency cesarean delivery. Journal of Maternal-Fetal & Neonatal Medicine 2015:1–4. [PubMed: 25946576]Elgafor 2013
Elgafor El Sharkwy IA. Carbetocin versus sublingual misoprostol plus oxytocin infusion for prevention of postpartum hemorrhage at cesarean section in patients with risk factors: a randomized, open trail study. Archives of Gynecology and Obstetrics 2013;288(6):1231–6. [PubMed: 23689739]El-Refaey
El-Refaey H, Nooh R, O'Brien P, Abdalla M, Geary M, Walder J, et al The misoprostol third stage of labour study: a randomised controlled comparison between orally administered misoprostol and standard management. BJOG: an international journal of obstetrics and gynaecology 2000;107:1104–10. [PubMed: 11002953]Elsedeek 2012
Elsedeek MS. Impact of preoperative rectal misoprostol on blood loss during and after elective cesarean delivery. International Journal of Gynecology & Obstetrics 2012;118(2):149–52. [PubMed: 22698700]Enakpene 2007
Enakpene, Morhason-Bello IO, Enakpene EO, Arowojolu AO, Omigbodun AO. Oral misoprostol for the prevention of primary post-partum hemorrhage during third stage of labor. Journal of Obstetrics and Gynaecology Research 2007;33(6):810–7. [PubMed: 18001447]Ezeama 2014
Ezeama CO, Eleje GU, Ezeama NN, Igwegbe AO, Ikechebelu JI, Ugboaja JO, et al A comparison of prophylactic intramuscular ergometrine and oxytocin for women in the third stage of labor. International Journal of Gynecology & Obstetrics 2014;124(1):67–71. [PubMed: 24365208]Fararjeh 2003
Fararjeh C, Gezer A, Cepni I, Benian A, Ocal P, Kosebay D. The efficacy of misoprostol in preventing postpartum bleeding [Postpartum Kanama Profilaksisinde Rektal Mizoprostol Kulanımının Etkinliği.]. Jinekoloji ve Obstetrik Dergisi 2003;17(4):218–23.Fawole 2011
Fawole AO, Sotiloye OS, Hunyinbo KI, Umezulike AC, Okunlola MA, Adekanle DA, et al A double-blind, randomized, placebo-controlled trial of misoprostol and routine uterotonics for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2011;112(2):107–11. [PubMed: 21130446]Fazel 2013
Fazel MR, Mansoure-Samimi, Esmaeil-Fakharian. A comparison of rectal misoprostol and intravenous oxytocin on hemorrhage and homeostatic changes during cesarean section. Middle East Journal of Anesthesiology 2013;22(1):41–6. [PubMed: 23833849]Fekih 2009
Fekih M, Jnifene A, Fathallah K, Ben Regaya L, Memmi A, Bouguizene S, et al Benefit of misoprostol for prevention of postpartum hemorrhage in cesarean section: a randomized controlled trial. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2009;38(7):588–93. [PubMed: 19833454]Fenix 2012
Fenix AM. Double-blind randomized controlled trial comparing the effect of carbetocin with oxytocin for the prevention of postpartum hemorrhage among high risk women following vaginal delivery. International Journal of Gynaecology & Obstetrics 2012;119(Suppl 3):S347–S348.Fu 2003
Fu YX, Ran KQ, Wang M. Prevention of early postpartum hemorrhage by way of oral misoprostol. Journal of Nursing Science 2003;18(12):910–1.Garg 2005
Garg P, Batra S, Gandhi G. Oral misoprostol versus injectable methylergometrine in management of the third stage of labor. International Journal of Gynecology & Obstetrics 2005;91(2):160–1. [PubMed: 16126208]Gavilanes 2016
Gavilanes P, Morales MF, Velasco S, Teran E. Sublingual misoprostol is as effective as intravenous oxytocin to reduce intra-operative blood loss during cesarean delivery in women living at high altitude. Journal of Maternal-Fetal & Neonatal Medicine 2016;29(4):559–61. [PubMed: 25666740]Gerstenfeld 2001
Gerstenfeld TS, Wing DA. Rectal misoprostol versus intravenous oxytocin for the prevention of postpartum hemorrhage after vaginal delivery. American Journal of Obstetrics and Gynecology 2001;185:878–82. [PubMed: 11641670]Guzmezoglu 2001
Gulmezoglu AM, Villar J, Ngoc NTN, Piaggio G, Carroli G, Adetoro L, et al WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet 2001;358:689–95. [PubMed: 11551574]- •Lumbiganon P, Hofmeyr J, Gulmezoglu AM, Pinol A, Villar J. Misoprostol dose-related shivering and pyrexia in the third stage of labour Who collaborative trial of misoprostol in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1999;106(4):304–8. [PubMed: 10426235]
- •Lumbiganon P, Villar J, Piaggio G, Gulmezoglu AM, Adetoro L, Carroli G. Side effects of oral misoprostol during the first 24 hours after administration in the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2002;109:1222–6. [PubMed: 12452458]
Gupta 2006
Gupta B, Jain V, Aggarwal N. Rectal misoprostol versus oxytocin in the prevention of postpartum hemorrhage - a pilot study. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S139–S140. [PubMed: 29644697]Hamm 2005
Hamm J, Russell Z, Botha T, Carlan SJ, Richichi K. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. American Journal of Obstetrics and Gynecology 2005;192:1404–6. [PubMed: 15902121]Harriott 2009
Harriott J, Christie L, Wynter S, DaCosta V, Fletcher H, Reid M. A randomized comparison of rectal misoprostol with syntometrine on blood loss in the third stage of labour. West Indian Medical Journal 2009;58(3):201–6. [PubMed: 20043525]Hofmeyr 1998
Hofmeyr GJ, Nikodem C, Jager M, Drakely A, Gilbart B. Oral misoprostol for labour third stage management: randomised assessment of side effects. Proceedings of the 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:53–4.- •Hofmeyr GJ, Nikodem VC, Jager M, Gelbart BR. A randomised placebo controlled trial of oral misoprostol in the third stage of labour. British Journal of Obstetrics and Gynaecology 1998;105(9):971–5. [PubMed: 9763047]
- •Hofmeyr GJ, Jager M, Rose L, Nikodem VC, Lawrie T. Misoprostol for third stage of labour management: a double blind, placebo controlled clinical trial. Proceedings of the 16th Conference on Priorities in Perinatal Care; 1997; South Africa. 1997:29–31.
Hofmeyr 2000
Hofmeyr GJ, Nikodem VC, Jager M, Drakely AJ. Side effects of oral misoprostol in the third stage of labour: a random allocation placebo controlled trial. Journal of Obstetrics & Gynaecology 2000;20(Suppl 1):S40–1.- •Hofmeyr GJ, Nikodem VC, Jager M, Drakely A. Side-effects of oral misoprostol in the third stage of labour--a randomised placebo-controlled trial. South African Medical Journal 2001;91(5):432–5. [PubMed: 11455810]
Hofmeyr 2011
Hofmeyr GJ, Fawole B, Mugerwa K, Godi NP, Blignaut Q, Mangesi L, et al Administration of 400mug of misoprostol to augment routine active management of the third stage of labor. International Journal of Gynecology and Obstetrics 2011;112(2):98–102. [PubMed: 21130990]- •NCT00124540. Misoprostol for preventing postpartum hemorrhage. clinicaltrials.gov/ct2/show/NCT00124540 Date first received: 26 July 2005.
Hoj 2005
Hoj L, Cardoso P, Nielsen BB, Hvidman L, Nielsen J, Aaby P. Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in Guinea-Bissau: randomised double blind clinical trial. BMJ 2005;331:723–7. [PMC free article: PMC1239973] [PubMed: 16195287]- •Nielsen BB, Hoj L, Hvidman LE, Nielsen J, Cardoso P, Aaby P. Reduced post-partum bleeding after treatment with sublingual misoprostol: a randomized double-blind clinical study in a developing country - secondary publication [Reduceret post partum-blodning efter sublingval misoprostol: et randomiseret dobbeltblindt klinisk studie i et udviklingsland - sekundaerpublikation]. Ugeskrift for Laeger 2006;168(13):1341–3. [PubMed: 16579891]
Hong 2007
Hong SC, Kim JW, Park HT, Seol HJ, Kim HJ, Kim SH, et al Additional rectal misoprostol plus intravenous oxytocin versus intravenous oxytocin for the prevention of postpartum hemorrhage after cesarean section. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S99, Abstract no: 321.Ibrahim 2017
Ibrahim KAAM SA. Prevention of postpartum haemorrhage in patients with severe preeclampsia using carbetocin versus misoprostol. Apollo Med 2017; 2(14): 117–122Ibrahim 2020
Ibrahim Zakia M, Sayed Ahmed, Waleed A, Abd El-Hamid, Eman M et al Carbetocin versus oxytocin for prevention of postpartum hemorrhage in hypertensive women undergoing elective cesarean section. Hypertension in pregnancy 2020; 39(3): 319–325 [PubMed: 32421401]Is 2012
Is S, Gr V, Keranahalli S. Comparison of intramuscular ergometrine and per rectal misoprostol for prophylaxis against atonic post patrum haemorrhage. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S797–S798.Jago 2007
Jago AA, Ezechi OC, Achinge GI, Okunlola MA. Effect of oxytocics on the blood pressure of normotensive Nigerian parturients. Journal of Maternal-Fetal & Neonatal Medicine 2007;20(9):703–5. [PubMed: 17701671]Jain 2019
Jain R., Agrawal S., Verma K. et al Comparison of intramuscular methylergometrine, rectal misoprostol, and low-dose intravenous oxytocin in active management of the third stage of labor. Tzu Chi Medical Journal 2019; 31(3): 158–162 [PMC free article: PMC6559031] [PubMed: 31258291]Jangsten 2011
Jangsten E, Mattsson L, Lyckestam I, Hellström A, Berg M. A comparison of active management and expectant management of the third stage of labour: a Swedish randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118:362–9. [PubMed: 21134105]Jerbi 2007
Jerbi M, Hidar S, Elmoueddeb, Chaieb A, Khairi H. Oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics 2007;96(3):198–9. [PubMed: 17289048]Jirakulsawas 2000
Jirakulsawas J, Khooarmompattana S. Comparison of oral misoprostol and intramuscular methylergonovine for prevention of postpartum hemorrhage. Thai Journal of Obstetrics and Gynaecology 2000;12(4):332.Kang 2022
Kang S., Zhou L., Zhu L. et al Carbetocin versus oxytocin for the prevention of postpartum hemorrhage after elective caesarean section in high risk women: a prospective, randomized, open-label, controlled trial in China. Clinical and Experimental Obstetrics and Gynecology 2022; 49(1): 023Karkanis 2002
Karkanis SG, Caloia D, Salenieks ME, Kingdom J, Walker M, Meffe F, et al Randomized controlled trial of rectal misoprostol versus oxytocin in third stage management. Journal of Obstetrics and Gynaecology Canada: JOGC 2002;24(2):149–54. [PubMed: 12196880]Kerekes 1979
Kerekes L, Domokos N. The effect of prostaglandin F2alpha on third stage labour. Prostaglandins 1979;18:161–6. [PubMed: 392622]Khan 1995
Khan GQ, John IS, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin vs syntometrine in the active management of the third stage of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;58:147–51. [PubMed: 7774741]- •Khan GQ, Susheela J I, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin versus syntometrine in the active management of the third stage of labour. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4–7; Dublin, Ireland. 1995:Abstract no: 212. [PubMed: 7774741]
Kikutani 2006
Kikutani T, Kikutani M, Oshima M, Sugimoto K, Shimada Y. Effects of methylergometrine and oxytocin on blood loss and uterine contraction during cesarean section. Masui - Japanese Journal of Anesthesiology 2006;55(5):590–4. [PubMed: 16715913]Kumar 2021
Kumar Burman S., Samanta R., Lata K.K. et al Prophylactic administration of per rectal misoprostol vs intramuscular injection of oxytocin in third-stage of labour for prevention of postpartum Haemorrhage: A randomised controlled trial. Journal of Clinical and Diagnostic Research 2021;15(9): qc09–qc13Kumru 2005
Kumru S, Gurates B, Parmaksiz C. Investigation of the usefulness of methyl ergonovine application in cesarean section cases [Sezaryen olgularinda metil ergonovin uygulamasinin yararliliginin arastirilmasi]. Journal of the Turkish German Gynecology Association Artemis 2005;6(1):42–5.Kundodyiwa 2001
Kundodyiwa TW, Majoko F, Rusakaniko S. Misoprostol versus oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics 2001;75:235–41. [PubMed: 11728483]Lam 2004
Lam H, Tang OS, Lee CP, Ho PC. A pilot-randomized comparison of sublingual misoprostol with syntometrine on the blood loss in third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 2004;83:647–50. [PubMed: 15225189]Lapaire 2006
Lapaire O, Schneider MC, Stotz M, Surbek DV, Holzgreve W, Hoesli IM. Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean delivery. International Journal of Gynecology & Obstetrics 2006;95(1):2–7. [PubMed: 16934269]Leung 2006
Leung SW, Ng PS, Wong WY, Cheung TH. A randomised trial of carbetocin versus syntometrine in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2006;113:1459–64. [PMC free article: PMC1804104] [PubMed: 17176279]Liu 2020
Liu H., Xu X.-Y., Gu N. et al Intravenous Administration of Carbetocin Versus Oxytocin for Preventing Postpartum Hemorrhage after Vaginal Delivery in High Risk Women: A Double-blind, Randomized Controlled Trial. Maternal-Fetal Medicine 2020; 2(2): 72–79Lokugamage 1999
Lokugamage AU, Moodley J, Sullivan K, Rodeck CH, Niculescu L, Tigere P. The Durban primary postpartum haemorrhage study. Women's Health - into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3–6; Cape Town South Africa. RCOG, 1999:77–8.- •Lokugamage AU, Paine M, Bassau-Balroop H, El-Refaey K, Sullivan K, Rodek C. Active management of the third stage at caesarean section: misoprostol vs syntocinon. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3–8; Washington DC, USA. 2000; Vol. Book 2:54.
- •Lokugamage AU, Paine M, Bassaw-Balroop K, Sullivan KR, El-Refaey H, CH Rodeck. Active management of the third stage at caesarean section: a randomised controlled trial of misoprostol versus syntocinon. Australian & New Zealand Journal of Obstetrics & Gynaecology 2001;41(4):411–4. [PubMed: 11787915]
Lumbiganon 1999
Lumbiganon P, Hofmeyr J, Gülmezoglu AM, Pinol A, Villar J. Misoprostol dose-related shivering and pyrexia inthe third stage of labour. British Journal of Obstetrics and Gynaecology 1999;106:304–8. [PubMed: 10426235]Maged 2016
Maged AM, Hassan AM, Shehata NA. Carbetocin versus oxytocin for prevention of postpartum hemorrhage after vaginal delivery in high risk women. Journal of Maternal-Fetal & Neonatal Medicine 2016;29(4):532–6. [PubMed: 25731657]Maged 2020
Maged AM, Waly M, Fahmy RM et al Carbetocin versus rectal misoprostol for management of third stage of labor among women with low risk of postpartum hemorrhage. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2020; 148(2): 238–242 [PubMed: 31736069]Masse 2022
Masse N; Dexter F; Wong CA. Prophylactic Methylergonovine and Oxytocin Compared With Oxytocin Alone in Patients Undergoing Intrapartum Cesarean Birth: A Randomized Controlled Trial. Obstetrics and gynecology 2022; 140(2): 181–186 [PubMed: 35852267]McDonagh 2022
McDonagh F, Carvalho J C A, Abdulla S et al Carbetocin vs. oxytocin at elective caesarean delivery: a double-blind, randomised, controlled, non-inferiority trial of low- and high-dose regimens. Anaesthesia 2022. [PubMed: 35343585]McDonald 1992
McDonald S. The Perth third stage oxytocic trial. Proceedings of National Conference on Research in Midwifery; 1992; Reading, UK. 1992.- •McDonald S, Prendiville WJ. A randomized controlled trial of syntocinon vs syntometrine as part of the active management of the third stage of labour. Journal of Perinatal Medicine 1992;20(Suppl 1):97.
- •McDonald S, Prendiville WJ, Blair E. A randomised controlled trial of syntocinon vs syntometrine as part of the active management of the third stage of labour. Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992 July 7–10; Manchester, UK. 1992:87.
- •McDonald SJ. Management in the third stage of labour [thesis]. University of Western Australia, 1996.
- •McDonald SJ, Prendiville WJ, Blair E. Randomised controlled trial of oxytocin alone vs oxytocin and ergometrine in active management of third stage of labour. BMJ 1993;307:1167–71. [PMC free article: PMC1679299] [PubMed: 8251842]
Mitchell 1993
Mitchell GG, Elbourne DR. The Salford third stage trial: oxytocin plus ergometrine vs oxytocin alone in the active management of the third stage of labor. Online Journal of Current Clinical Trials 1993;2:Doc 83. [PubMed: 8306013]Mobeen 2011
Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo-controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118:353–61. [PMC free article: PMC3041931] [PubMed: 21176086]- •Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al Use of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage during home deliveries in Pakistan: a randomised placebo-controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S92. [PMC free article: PMC3041931] [PubMed: 21176086]
- • NCT00120237. Misoprostol for the prevention of postpartum hemorrhage in rural Pakistan. clinicaltrials.gov/ct2/show/NCT00120237 Date first received: 7 July 2005.
Moertl 2008
Moertl M, Kraschl J, Friedrich S, Pickel K, Ulrich D, Eder M, et al Hemodynamic changes of carbetocin and oxytocin in women undergoing cesarean section. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S112.- •Moertl MG, Friedrich S, Kraschl J, Wadsack C, Lang U, Schlembach D. Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2011;118(11):1349–56. [PubMed: 21668768]
- •Mortl M, Pickel K, Friedrich S, Ulrich D, Lang U, Schlembach D. Hemodynamic changes of carbetocin and oxytocin given as i.v. bolus on women undergoing cesarean section. Geburtshilfe und Frauenheilkunde 2008;68:S46.
Moir 1979
Moir DD, Amoa AB. Ergometrine or oxytocin? Blood loss and side-effects at spontaneous vertex delivery. British Journal of Anaesthesia 1979;51(2):113–7. [PubMed: 371650]Moodie 1976
Moodie JE, Moir DD. Ergometrine, oxytocin and extradural analgesia. British Journal of Anaesthesia 1976;48:571–4. [PubMed: 952692]Mukta 2013
Mukta M, Sahay PB. Role of misoprostol 600 mcg oral in active management of third stage of labor: a comparative study with oxytocin 10 IU i.m. Journal of Obstetrics and Gynecology of India 2013;6:325–7. [PMC free article: PMC3798442] [PubMed: 24431668]Musa 2015
Musa AO, Ijaiya MA, Saidu R, Aboyeji AP, Jimoh AA, Adesina KT, et al Double-blind randomized controlled trial comparing misoprostol and oxytocin for management of the third stage of labor in a Nigerian hospital. International Journal of Gynecology & Obstetrics2015; Vol. 129, issue 3:227–30. [PubMed: 25835642]Nahaer 2020
Nahaer M.K., Nurunnobi A.K.M., Talukder S.I. et al Carbetocin versus oxytocin for prophylaxis of PPH used during caesarean section: An open label randomized control trial. Bangladesh Journal of Obstetrics and Gynecology 2020; 33(2): 113–118Nasr 2009
Nasr A, Shahin AY, Elsamman AM, Zakherah MS, Shaaban OM. Rectal misoprostol versus intravenous oxytocin for prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2009;105(3):244–7. [PubMed: 19249048]Ng 2001
Ng PS, Chan ASM, Sin WK, Tang LCH, Cheung KB, Yuen PM. A multicentre randomized controlled trial of oral misoprostol and im syntometrine in the management of the third stage of labour. Human Reproduction 2001;16(1):31–5. [PubMed: 11139532]- •Ng PS, Chan ASM, Sin WK, Tang LCH, Cheung KB, Yuen PM. Comparison of oral misoprostol and intramuscular syntometrine in the management of the third stage of labor - a multicenter randomised controlled trial. XVI FIGO World Congress of Obstetrics & Gynecology. Book 4; 2000 Sept 3–8; Washington DC, USA. 2000:29.
Ng 2007
Ng PS, Lai CY, Sahota DS, Yuen PM. A double-blind randomized controlled trial of oral misoprostol and intramuscular syntometrine in the management of the third stage of labor. Gynecologic and Obstetric Investigation 2007;63(1):55–60. [PubMed: 16940738]- •Yuen PM, Ng PS, Sahota DS. A double-blind randomised controlled trial of oral misoprostol in addition to intra-muscular syntometrine in the management of the third stage of labour. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7–9; Glasgow, UK. 2004:62.
Nihar 2022
Nihar S., Mukherjee S., Banumathy et al Comparative study of blood loss in C-section with usage of intravenous oxytocin and intramuscular methergine-in a tertiary care hospital. Journal of Cardiovascular Disease Research 2022; 13(3): 1–9Nirmala 2009
Nirmala K, Zainuddin AA, Ghani NA, Zulkifli S, Jamil MA. Carbetocin versus syntometrine in prevention of post-partum hemorrhage following vaginal delivery. Journal of Obstetrics and Gynaecology Research 2009;35(1):48–54. [PubMed: 19215547]Nordstrom 1997
Nordstrom L, Fogelstam K, Fridman G, Larsson A, Rydhstroem H. Routine oxytocin in the third stage of labour: a placebo controlled randomised trial. British Journal of Obstetrics and Gynaecology 1997;104(7):781–6. [PubMed: 9236641]Oboro 2003
Oboro VO, Tabowei TO. A randomised controlled trial of misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Obstetrics & Gynaecology 2003;23(1):13–6. [PubMed: 12623474]Ogunbode 1979
Ogunbode O, Obisesan K, Ayeni O. Methergin in the management of the third stage of labor: a comparative clinical trial with syntometrine and ergometrine. Current Therapeutic Research, Clinical and Experimental 1979;26:460–5.Orji 2008
Orji E, Agwu F, Loto O, Olaleye O. A randomized comparative study of prophylactic oxytocin versus ergometrine in the third stage of labor. International Journal of Gynecology & Obstetrics 2008;101(2):129–32. [PubMed: 18164304]Ortiz-Gomez 2013
Ortiz-Gomez JR, Morillas-Ramirez F, Fornet-Ruiz I, Palacio-Abizanda FJ, Bermejo-Albares L. [Clinical and pharmacological study of the efficacy of carbetocin in elective caesareans compared to low and usual doses of oxytocin]. [Spanish]. Revista Espanola de Anestesiologia y Reanimacion 2013;60(1):7–15. [PubMed: 23122840]Otoide 2020
Otoide VO A double blind randomized controlled clinical trial of oral misoprostol versus ergometrine in the prevention of primary postpartum hemorrhage. Tropical journal of obstetrics and gynaecology 2020; 37(1): 72–77Ottun 2021
Ottun Tawakwallit A, Adewunmi Adeniyi A, Rabiu Afolarin K et al Misoprostol and oxytocin versus oxytocin alone in the active management of the third stage of labour: a randomised, double-blind, placebo-controlled trial. Journal of obstetrics and gynaecology: the journal of the Institute of Obstetrics and Gynaecology 2021; 1–6 [PubMed: 34958620]Owonikoko 2011
Owonikoko KM, Arowojolu AO, Okunlola MA. Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at cesarean section in Nigeria: A randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2011;37(7):715–21. [PubMed: 21375669]Parson 2006
Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Oral misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology Canada 2006;28(1):20–6. [PubMed: 16533451]Parsons 2004
Parsons S, Ntumy YM, Walley RL, Wilson JB, Crane JM G, Matthews K, et al Rectal misoprostol vs intramuscular oxytocin in the routine management of the third stage of labour. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7–9; Glasgow, UK. 2004:18.- •Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Rectal misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology Canada 2007;29(9):711–8. [PubMed: 17825135]
Penaranda 2002
Penaranda WA, Arrieta OB, Yances BR. Active management of childbirth with sublingual misoprostol: a controlled clinical trial in the Hospital de Maternidad Rafael Calvo [Manejo activo del alumbramiento con misoprostol sublingual: un estudio clinico controlado en al hospital de maternidad rafael calvo de cartagena]. Revista Colombiana de Obstetricia y Ginecologia 2002;53(1):87–92.Prendiville 1988
Prendiville WJ, Harding JE, Elbourne DR, Stirrat GM. The Bristol third stage trial: active versus physiological management of third stage of labour. BMJ 1988;297(6659):1295–300. [PMC free article: PMC1834913] [PubMed: 3144366]Rajaei 2014
Rajaei M, Karimi S, Shahboodaghi Z, Mahboobi H, Khorgoei T, Rajaei F. Safety and efficacy of misoprostol versus oxytocin for the prevention of postpartum hemorrhage. Journal of Pregnancy 2014;2014:713879. [PMC free article: PMC3964754] [PubMed: 24734184]Ramirez 2001
Ramirez O, Benito V, Jimenez R, Valido C, Hernandez C, Garcia JA. Third stage of labour: active or expectant management? preliminary results [abstract]. Journal of Perinatal Medicine 2001;Suppl 1(Pt 2):364.Rashid 2009
Rashid M, Clark A, Rashid MH. A randomised controlled trial comparing the efficacy of intramuscular syntometrine and intravenous syntocinon, in preventing postpartum haemorrhage. Journal of Obstetrics and Gynaecology 2009;29(5):396–401. [PubMed: 19603316]Ray 2001
Ray A, Mukherjee P, Basu G, Chatterjee A. Misoprostol and third stage of labour. Journal of Obstetrics and Gynecology of India 2001;51(6):53–4.Reyes 2011
Reyes OA, Gonzalez GM. Carbetocin versus oxytocin for prevention of postpartum hemorrhage in patients with severe preeclampsia: a double-blind randomized controlled trial. Journal of Obstetrics and Gynaecology Canada: JOGC 2011;33(11):1099–104. [PubMed: 22082783]Reyes 2011
Reyes OA. Carbetocin vs. oxytocin for the prevention of postpartum hemorrhage grand multipara patients: randomized controlled trial [Carbetocina vs. oxitocina para la prevención de hemorragia posparto en pacientes grandes multíparas: estudio aleatorizado controlado]. Clinica e Investigacion en Ginecologia y Obstetricia 2011;38:2–7.Rogers 1998
Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial. Lancet 1998;351(9104):693–9. [PubMed: 9504513]Rosseland 2013
Rosseland LA, Hauge TH, Grindheim G, Stubhaug A, Langesaeter E. Changes in blood pressure and cardiac output during cesarean delivery: the effects of oxytocin and carbetocin compared with placebo. Anesthesiology 2013;119(3):541–51. [PubMed: 23598289]- •Gawecka E, Rosseland LA. A secondary analysis of a randomized placebo-controlled trial comparing the analgesic effects of oxytocin with carbetocin: postcesarean delivery morphine equivalents. Anesthesia and Analgesia 2014;119(4):1004. [PubMed: 25232698]
Rozenberg 2015
Rozenberg P, Quibel T, Ghout I, Salomon L, Bussiere L, Goffinet F. Active management of the third stage of labor with routine oxytocin and misoprostol for the prevention of postpartum hemorrhage: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S18. [PubMed: 27607864]Sadiq 2011
Sadiq UG, Kwanashie O, Mairiga G, Gamaniel S, Isa H, Abdu A, et al A randomised clinical trial comparing the efficacy of oxytocin injection and oral misoprostol tablet in the prevention of postpartum haemorrhage in Maiduguri Nigeria. International Research Journal of Pharmacy 2011;2(8):76–81.Samimi 2013
Samimi M, Imani-Harsini A, Abedzadeh-Kalahroudi M. Carbetocin vs. syntometrine in prevention of postpartum hemorrhage: a double blind randomized control trial. Iranian Red Crescent Medical Journal 2013;15(9):817–22. [PMC free article: PMC3929818] [PubMed: 24616793]Shady 2019
Shady Nahla W, Sallam Hany F, Elsayed Ahmed H et al. The effect of prophylactic oral tranexamic acid plus buccal misoprostol on blood loss after vaginal delivery: a randomized controlled trial. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians 2019; 32(11): 1806–1812 [PubMed: 29241383]Shaheen 2019
Shaheen N. and Khalil S. Safety and efficacy of 600ug sublingual misoprostol versus 10 U intramuscular Oxytocin for management of third stage of labor. Rawal Medical Journal 2019; 44(1): 137–140Shrestha 2011
Shrestha A, Dongol A, Chawla CD, Adhikari RK. Rectal misoprostol versus intramuscular oxytocin for prevention of post partum hemorrhage. Kathmandu University Medical Journal 2011;33(1):8–12. [PubMed: 22610801]Singh 2009
Singh G, Radhakrishnan G, Guleria K. Comparison of sublingual misoprostol, intravenous oxytocin, and intravenous methylergometrine in active management of the third stage of labor. International Journal of Gynecology & Obstetrics 2009;107(2):130–4. [PubMed: 19628206]Soltan 2007
Soltan MH, El-Gendi E, Imam HH, Fathi O. Different doses of sublingual misoprostol versus methylergometrine for the prevention of atonic postpartum haemorrhage. International Journal of Health Sciences 2007;1(2):229–36. [PMC free article: PMC3068636] [PubMed: 21475433]Sood 2012
Sood AK, Singh S. Sublingual misoprostol to reduce blood loss at cesarean delivery. Journal of Obstetrics and Gynaecology of India 2012;62(2):162–7. [PMC free article: PMC3425684] [PubMed: 23543254]Stanton 2012
Stanton CK, Newton S, Mullany LC, Cofie P, Agyemang CT, Adiibokah E, et al Impact on postpartum hemorrhage of prophylactic administration of oxytocin 10 IU via Uniject by peripheral health care providers at home births: Design of a community-based cluster-randomized trial. BMC Pregnancy and Childbirth 2012;12:42. [PMC free article: PMC3406972] [PubMed: 22676921]- •NCT01108302. Effectiveness, safety and feasibility of auxiliary nurse midwives' (ANM) use of oxytocin in uniject™ to prevent postpartum hemorrhage in India. clinicaltrials.gov/ct2/show/NCT01108302 Date first received: 2 April 2010.
- •Stanton CK, Newton S, Mullany LC, Cofie P, Tawiah Agyemang C, Adiibokah E, et al Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community-based, cluster-randomized trial. PLoS Medicine. NCT01108289 2013; Vol. 10, issue 10:e1001524. [PMC free article: PMC3794862] [PubMed: 24130463]
Su 2009
Su LL, Rauff M, Chan YH, Mohamad Suphan N, Lau TP, Biswas A, et al Carbetocin versus syntometrine for the third stage of labour following vaginal delivery--a double-blind randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2009;116(11):1461–6. [PubMed: 19538418]Sultana 2007
Sultana N, Khatun M. Misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Bangladesh College of Physicians and Surgeons 2007;25(2):73–6.Surbek 2000
Surbek DV, Fehr PM, Hoesli I, Holzgreve W. Misoprostol for prevention of postpartum hemorrhage: a randomized controlled trial [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3–8; Washington DC, USA 2000;Book 1:33.- •Surbek DV, Fehr PM, Hoesli I, Holzgreve W. Oral misoprostol vs placebo for third stage of labour [Orales misoprostol reduziert den postpartalen blutverlust]. Gynakologisch Geburtshilfliche Rundschau 1999;39:144.
Sweed 2018
Sweed Mohamed S, El-Saied Mourad M, Abou-Gamrah Amgad E et al Rectal vs. sublingual misoprostol before cesarean section: double-blind, three-arm, randomized clinical trial. Archives of gynecology and obstetrics 2018; 298(6): 1115–1122 [PubMed: 30291483]Tahan 2012
Tahan MR, Warda OM, Rashad A, Yasseen AM, Ramzy EA, Ahmady MS, et al Effects of preoperative sublingual misoprostol on uterine tone during isoflurane anesthesia for cesarean section. Revista Brasileira de Anestesiologia 2012;62(5):625–35. [PubMed: 22999396]Tewatia 2014
Tewatia R, Rani S, Srivastav U, Makhija B. Sublingual misoprostol versus intravenous oxytocin in prevention of post-partum hemorrhage. Archives of Gynecology and Obstetrics 2014;289:739–42. [PubMed: 24045979]Thilaganathan 1993
Thilaganathan B, Cutner A, Latimer J, Beard R. Management of the third stage of labour in women at low risk of postpartum haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48:19–22. [PubMed: 8449257]Ugwu 2014
Ugwu IA, Enabor OO, Adeyemi AB, Lawal OO, Oladokun A, Olayemi O. Sublingual misoprostol to decrease blood loss after caesarean delivery: a randomised controlled trial. Journal of Obstetrics and Gynaecology 2014;34(5):407–11. [PubMed: 24724983]Uncu 2015
Uncu Y, Karahasan M, Uyaniklar O, Uncu G. Prophylactic misoprostol for the prevention of postpartum hemorrhage: a randomized controlled trial. European Review for Medical and Pharmacological Sciences 2015;19(1):15–22. [PubMed: 25635970]Un Nisa 2012
Un Nisa S, Usmani SY. Role of intravenous syntocinon in prevention of primary postpartum haemorrhage. Pakistan Journal of Medical and Health Sciences 2012;6(4):1020–4.Vagge 2013
Vagge DS, Mamatha KR, Rohatgi V. A comparative study to assess the efficacy and tolerability of per rectal misoprostol versus intravenous oxytocin in prevention of primary postpartum haemorrhage in a tertiary care hospital. Indian Journal of Pharmacology 2013;45 Suppl:S45.- •Vagge DS, Mamatha KR, Shivamurthy G, Rohatgi V. A comparative study to assess the efficacy and tolerability of per rectal misoprostol and intravenous oxytocin in prevention of primary postpartum haemorrhage in a tertiary care hospital. Journal of Chemical and Pharmaceutical Research 2014;6(3):1134–40.
Vaid 2003
Vaid A, Dadhwal V, Mittal S, Deka D, Misra R, Sharma JB, et al A randomized controlled trial of prophylactic sublingual misoprostol versus intramuscular methyl-ergometrine versus intramuscular 15-methyl PGF2alpha in active management of third stage of labor. Archives of Gynecology and Obstetrics 2009;280(6):893–7. [PubMed: 19277690]Van Der Nelson 2021
Van Der Nelson H., O'Brien S., Burnard S. et al Intramuscular oxytocin versus syntometrine versus carbetocin for prevention of primary postpartum haemorrhage after vaginal birth: A randomised double-blinded clinical trial of effectiveness, side effects and quality of life. BJOG: An International Journal of Obstetrics and Gynaecology 2021; 128(8): e31 [PubMed: 33300296]Verma 2006
Verma P, Aggarwal N, Jain V, Suri V. A double-blind randomized controlled trial to compare sublingual misoprostol with methylergometrine for prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S137–S138. [PubMed: 29644683]Vimala 2004
Vimala N, Mittal S, Kumar S, Dadhwal V, Mehta S. Sublingual misoprostol versus methylergometrine for active management of third stage of labor. International Journal of Gynecology & Obstetrics 2004;87:1–5. [PubMed: 15464767]Vimala 2006
Vimala N, Mittal S, Kumar S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. International Journal of Gynecology & Obstetrics 2006;92(2):106–10. [PubMed: 16343498]Walley 2000
Walley RL, Wilson JB, Crane JM, Matthews K, Sawyer E, Hutchens D. A double-blind placebo controlled randomised trial of misoprostol and oxytocin in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1111–5. [PubMed: 11002954]Whigham 2014
Whigham C, Gorelik A, Loughnan T, Trivedi A. Carbetocin versus oxytocin in active labour. BJOG: An International Journal of Obstetrics and Gynaecology 2014;121(Suppl 2):88.Yesim 2022
Yesmin S., Begum F., Bain S. et al Carbetocin versus Oxytocin in the Prevention of Postpartum Haemorrhage after Caesarean Section. Bangladesh Journal of Obstetrics and Gynecology 2022; 35(2): 63–67Yuen 1995
Yuen PM, Chan NST, Yim SF, Chang AMZ. A randomised double blind comparison of syntometrine and syntocinon in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1995;102:377–80. [PubMed: 7612530]Zachariah 2006
Zachariah ES, Naidu M, Seshadri L. Oral misoprostol in the third stage of labor. International Journal of Gynecology & Obstetrics 2006;92(1):23–6. [PubMed: 16271721]Zgaya 2020
Zgaya R., Ghadhab I., Triki M.A. et al Randomized controlled trial comparing 400mug sublingual misoprostol versus placebo for prevention of primary postpartum hemorrhage. Pan African Medical Journal 2020; 36: 1–9 [PMC free article: PMC7467627] [PubMed: 32952830]Economic
Gallos 2019
Gallos I, Williams H, Price M et al Uterotonic drugs to prevent postpartum haemorrhage: a network meta-analysis. Health Technol Assess. 2019 Feb;23(9):1–356. [PMC free article: PMC6421507] [PubMed: 30821683]Matthijsse 2022
Matthijsse S, Andersson FL, Gargano M et al Cost-effectiveness analysis of carbetocin versus oxytocin for the prevention of postpartum hemorrhage following vaginal birth in the United Kingdom. J Med Econ. 2022 Jan-Dec;25(1):129–137. [PubMed: 35007465]
Effectiveness
Main references are in bold, followed by additional references to the trial
Appendices
Appendix A. Review protocols
Appendix B. Literature search strategies
Appendix C. Effectiveness evidence study selection
Study selection for: What is the effectiveness of uterotonics for the prevention of postpartum haemorrhage? (PDF, 186K)
Appendix D. Evidence tables
Evidence tables for review question: What is the effectiveness of uterotonics for the prevention of postpartum haemorrhage?
Due to the size and complexity of these tables they are provided in a separate document. See Supplement 4.
Appendix E. Forest plots
Appendix F. GRADE tables
GRADE tables for review question: What is the effectiveness of uterotonics for the prevention of postpartum haemorrhage?
Due to the size and complexity of these tables they are provided in a separate document. See Supplement 5.
Appendix G. Economic evidence study selection
Study selection for: What is the effectiveness of uterotonics for the prevention of postpartum haemorrhage? (PDF, 160K)
Appendix H. Economic evidence tables
Appendix I. Economic model
Appendix J. Excluded studies
Excluded studies for review question: What is the effectiveness of uterotonics for the prevention of postpartum haemorrhage?
Excluded effectiveness studies
Table 47Excluded studies and reasons for their exclusion. From the update search
| Study | Reason |
|---|---|
| Carbetocin Versus Buccal Misoprostol Plus IV Tranexamic Acid for Prevention of Postpartum Hemorrhage at Cesarean Section. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Buccal Misoprostol Versus IV Oxytocin in Prevention of Postpartum Hemorrhage. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Carbetocin Versus Ergometrin in the Prevention of Postpartum Hemorrhage. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Carbetocin Versus Oral Tranexamic Acid Plus, Buccal Misoprostol on Blood Loss After Vaginal Delivery. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Carbetocin Versus Oxytocin and Ergometrine for the Prevention of Postpartum Hemorrhage. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Carbetocin Versus Oxytocin for Prevention of Postcesarean Hemorrhage in Pregnancy With High Risk for PPH. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Carbetocin Versus Oxytocin for the Prevention of Postpartum Hemorrhage in Emergency Caesarean Delivery. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Carbetocin Versus Oxytocin Infusion Plus Tranexamic Acid During Cesarean Section. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Carbetocin Versus Oxytocin Plus Sublingual Misoprostol in the Management of Atonic Postpartum Hemorrhage. |
- Study design not in PICO Network meta analysis - checked for eligible studies |
| Carbetocin Versus Syntocinon for Prevention of Postpartum Hemorrhage in Cardiac Patients Undergoing Caesarean Section. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Carbetocin Versus Syntometrine in Obese Women Undergoing Elective Cesarean. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| The Comparison of the Effect of Different Oxytocin Administrations on the Blood Loss During Cesarean Delivery. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Double Simultaneous Uterotonic Agents Versus Single Agent Regimen to Prevent Early Postpartum Hemorrhage. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| The Effect of Labor Induction With Oxytocin on Early Postpartum Hemorrhage, Perineal Integrity and Breastfeeding. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Misoprostol Before and After Cesarean Section. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Oxytocin at Elective Cesarean Deliveries: A Dose-finding Study in Women With BMI ≥ 40kg/m2. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Oxytocin i.m./i.v. Versus Carbetocin i.v. in Elective Cesarean Sections. |
- Study design not in PICO Clinical trial - No results posted or publication provided |
| Oxytocin Versus, Sublingual Misoprostol in the Secondary Prevention of Postpartum Hemorrhage After Vaginal Delivery. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Oxytocin vs Carbetocin at Cesarean Delivery in Women With Morbid Obesity. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Preoperative and Postoperative Sublingual Misoprostol for Prevention of Postpartum Blood Loss in Cesarean Section. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Prevention of Primary Postpartum Haemorrhage. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Second-Line Uterotonics in Postpartum Hemorrhage: A Randomized Clinical Trial. |
- Duplicate Duplicate from IS search |
| Sublingual Misoprostol With or Without Intravenous Tranexamic Acid During Hemorrhagic Cesarean Section. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Sublinaual vs Intrauterine MISOPROSTOL Plus Oxytocin Infusion for Prevention of Post-cesarean Hemorrhage in High Risk Pregnant Women: A Double-blind Placebo RCT. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| A Trial of Sublingual Misoprostol to Reduce Primary Postpartum Haemorrhage After Vaginal Delivery. |
- Study design not in PICO Clinical trial - no results posted or publication provided |
| Carbetocin at Elective Cesarean Deliveries: A Dose-finding Study in Women With BMI ≥ 40kg/m2. |
- Unavailable from IS search Journal information unavailable so could no be found |
| Carbetocin Versus Rectal Misoprostol for Management of Third Stage of Labor in Women at Low Risk of Postpartum Hemorrhage. |
- Unavailable from IS search Journal information unavailable so could no be found |
| The Effect of Preoperative and Post Operative Misoprostol Administration on Intraoperative Blood Loss and Postpartum Hemorrhage in CS. |
- Unavailable from IS search Journal information unavailable so could no be found |
| Intrauterine Misoprostol Versus Rectal Misoprostol in Reducing Blood Loss During Cesarean Section. |
- Unavailable from IS search Journal information unavailable so could no be found |
| Randomization of Oxytocin, Oxytocin+Intrauterine Misoprostol and Carbetocin During C-section. |
- Unavailable from IS search Journal information unavailable so could no be found |
| Multimodal Uterotonics at the Time of Cesarean Section in Laboring Patients. |
- Study design not in PICO Conference abstract |
| Abbas, Dina F, Mirzazada, Shafiq, Durocher, Jill et al (2020) Testing a home-based model of care using misoprostol for prevention and treatment of postpartum hemorrhage: results from a randomized placebo-controlled trial conducted in Badakhshan province, Afghanistan. Reproductive health 17(1): 88 [PMC free article: PMC7275481] [PubMed: 32503556] |
- Intervention not in PICO Intervention for treatment of PPH |
| Abdelaleem, Ahmed A, Abbas, Ahmed M, Thabet, Andrew L et al (2019) The effect of initiating intravenous oxytocin infusion before uterine incision on the blood loss during elective cesarean section: a randomized clinical trial. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians 32(22): 3723–3728 [PubMed: 29712515] |
- Intervention not in PICO Only one uterotonic - intravenous oxytocin infusion before uterine incision versus late after umbilical cord clamping |
| Adnan, Nita, Conlan-Trant, Rebecca, McCormick, Ciara et al (2018) Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomised controlled trial. BMJ (Clinical research ed.) 362: k3546 [PMC free article: PMC6122278] [PubMed: 30181338] |
- Intervention not in PICO Same drug intervention both arms only different routes of administration |
| Ahmadi, Fatemeh (2018) A comparative study on infusion of usual dose of oxytocin and 80 units dose of oxytocin in the prevention of postpartum hemorrhage in cesarean section. Journal of advanced pharmaceutical technology & research 9(3): 102–106 [PMC free article: PMC6174700] [PubMed: 30338236] |
- Intervention not in PICO Only one uterotonic - different doses of oxytocin |
| Alalfy, Mahmoud, Lasheen, Yossra, Elshenoufy, Hossam et al (2020) The efficacy of intrauterine misoprostol during cesarean section in prevention of primary PPH, a randomized controlled trial. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians 33(9): 1459–1465 [PubMed: 30176758] |
- Non systemic administration Intrauterine misoprostol |
| Anger, Holly A, Dabash, Rasha, Hassanein, Nevine et al (2020) A cluster-randomized, non-inferiority trial comparing use of misoprostol for universal prophylaxis vs. secondary prevention of postpartum hemorrhage among community level births in Egypt. BMC preanancy and childbirth 20(1): 317 [PMC free article: PMC7245883] [PubMed: 32448257] |
- Intervention not in PICO Only one uterotonic - 600mcg oral misoprostol versus 800mcg sublingual misoprostol Primary vs secondary prevention (i.e. provided only to women with postpartum blood loss) |
| Ashwal, Eran, Amikam, Uri, Wertheimer, Avital et al (2022) Route of postpartum oxytocin administration and maternal hemoglobin decline - A randomized controlled trial. European journal of obstetrics, gynecology, and reproductive biology 272: 134–138 [PubMed: 35305347] |
- Intervention not in PICO Only one uterotonic - 1) Intramuscular 10units; 2) intravenous 10units in 100 ml 0.9%NaCl solution over 10–15 min; 3) combined IV + IM regimens |
| Awoleke, J.O., Adeyanju, B.T., Adeniyi, A. et al (2020) Randomised Controlled Trial of Sublingual and Rectal Misoprostol in the Prevention of Primary Postpartum Haemorrhage in a Resource-Limited Community. Journal of Obstetrics and Gynecology of India 70(6): 462–470 [PMC free article: PMC7758385] [PubMed: 33417650] |
- Intervention not in PICO Same drug intervention both arms only different routes of administration - 600 mcg of misoprostol rectally or 600 mcg of misoprostol sublingually |
| Bahadur, Anupama, Khoiwal, Kavita, Bhattacharya, Namrata et al (2019) The effect of intrauterine misoprostol on blood loss during caesarean section. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology 39(6): 753–756 [PubMed: 31010345] |
- Non systemic administration Intrauterine misoprostol |
| Baliuliene, Vilda; Vitartaite, Migle; Rimaitis, Kestutis (2021) Prophylactic Dose of Oxytocin for Uterine Atony during Caesarean Delivery: A Systematic Review. International journal of environmental research and public health 18(9) [PMC free article: PMC8126197] [PubMed: 34068723] |
- Intervention not in PICO Same drug intervention both arms but different IV doses of oxytocin |
| Barua, HR, Barua, R, Barua S, EA (2017) Carbetocin and oxytocin in the active management of third stage of labor after vaginal birth of baby. Bangladesh Med J 1(46): 7–10 |
- Study design not in PICO Not a randomised trial |
| Begley, Cecily M, Gyte, Gillian MI, Devane, Declan et al (2019) Active versus expectant management for women in the third stage of labour. The Cochrane database of systematic reviews 2: cd007412 [PMC free article: PMC6372362] [PubMed: 30754073] |
- Systematic review Checked for eligible studies - eligible studies included. Ineligible studies either did not meet inclusion criteria or already included in Gallos 2018 |
| Beiranvand, S, Karimi, A, Vahabi, S et al (2019) Comparison of the Mean Minimum Dose of Bolus Oxytocin for Proper Uterine Contraction during Cesarean Section. Current clinical pharmacology 14(3): 208–213 [PubMed: 31124424] |
- Study design not in PICO Cross sectional study |
| Biradar, A.M., Yaliwal, R.G., Kori, S.S. et al (2021) Randomised control trial of 3 iu intravenous oxytocin bolus with 7 iu oxytocin infusion versus 10 iu intramuscular oxytocin in the third stage of labour in the prevention of postpartum hemorrhage. International Journal of Women's Health and Reproduction Sciences 9(3): 171–175 |
- Intervention not in PICO Same drug intervention all arms but different routes of administration and different doses - 3 IU IV bolus and 7 IU infusion of oxytocin or 10 IU of IM oxytocin |
| Caceda, Sonia Indacochea: Ramos, Richard Rubio, Saborido, Carlos, Martin (2018) Pharmacoeconomic study comparing carbetocin with oxytocin for the prevention of hemorrhage following cesarean delivery in Lima, Peru. Journal of comparative effectiveness research 7(1): 49–55 [PubMed: 29264934] |
- Study design not in PICO Pharmacoecnomic study |
| Carroli, G., Durocher, J., Dzuba, I. et al (2018) Does route matter? intravenous versus intramuscular oxytocin for prevention of postpartum hemorrhage. International Journal of Gynecology and Obstetrics 143(supplement3): 236 |
- Intervention not in PICO Only one uterotonic - 10 IU oxytocin-IV versus IM-and a matching ampoule (saline) |
| Cecilia, Maria, Vilayaselvi, Reeta, Bansal, Ramandeep et al (2018) Ten units intravenous oxytocin over 2–4 h is as effective as 30 units over 8–12 h in preventing postpartum hemorrhage after cesarean section: A randomized controlled trial. Indian journal of pharmacology 50(5): 279–283 [PMC free article: PMC6302697] [PubMed: 30636832] |
- Intervention not in PICO Only one uterotonic - single-dose intravenous oxytocin over 2–4 h (total = 10 units) versus oxytocin maintenance infusion for 8–12 h (total = 30 units) |
| Charles, D, Anger, H, Dabash, R et al (2019) Intramuscular injection, intravenous infusion, and intravenous bolus of oxytocin in the third stage of labor for prevention of postpartum hemorrhage: a three-arm randomized control trial. BMC pregnancy and childbirth 19(1): 38 [PMC free article: PMC6339323] [PubMed: 30658605] |
- Intervention not in PICO Same drug intervention both arms only different routes of administration - 10 IU oxytocin administered as either IM injection; IV infusion or IV bolus |
| Charles, Dyanna, Anger, Holly, Dabash, Rasha et al (2019) Intramuscular injection, intravenous infusion, and intravenous bolus of oxytocin in the third stage of labor for prevention of postpartum hemorrhage: a three-arm randomized control trial. BMC pregnancy and childbirth 19(1): 38 [PMC free article: PMC6339323] [PubMed: 30658605] |
- Duplicate Duplicate from IS search |
| Drew, T, Balki, M, Farine, D et al (2020) Carbetocin at elective caesarean section: a sequential allocation trial to determine the minimum effective dose in obese women. Anaesthesia 75(3): 331–337 [PubMed: 31867715] |
- Study design not in PICO Dose finding study - no comparative group |
| Durocher, Jill, Dzuba, Ilana G, Carroli, Guillermo et al (2019) Does route matter? Impact of route of oxytocin administration on postpartum bleeding: A double-blind, randomized controlled trial. PloS one 14(10): e0222981 [PMC free article: PMC6772050] [PubMed: 31574114] |
- Intervention not in PICO Only one uterotonic - 10 IU oxytocin via IV infusion versus IM injection and a matching saline ampoule |
| Ebada, Mahmoud Ahmed; Elmatboly, Abdelmagid M; Baligh, Galal (2020) Intravenous Oxytocin versus Intramuscular Oxytocin for the Management of Postpartum Hemorrhage: A Systematic Review and Meta-Analysis. Current drug research reviews 12(2): 150–157 [PubMed: 32600245] |
- Intervention not in PICO One uterotonic only - IV versus IM oxytocin |
| El-Sherbini, Moutaz M, Maged, Ahmed M, Helal, Omneya M et al (2021) A comparative study between preoperative rectal misoprostol and intraoperative intrauterine administration in the reduction of blood loss during and after cesarean delivery: A randomized controlled trial. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 153(1): 113–118 [PubMed: 33064852] |
- Non systemic administration Rectal misoprostol versus intrauterine misoprostol |
| Feduniw, S, Warzecha, D, Szymusik, I et al (2020) Epidemiology, prevention and management of early postpartum hemorrhage - a systematic review. Ginekologia polska 91(1): 38–44 [PubMed: 32039467] |
- Systematic review Checked for eligible studies - eligible studies included. Ineligible studies either did not meet inclusion criteria or already included in Gallos 2018 |
| Gallos, Ioannis, Williams, Helen, Price, Malcolm et al (2019) Uterotonic drugs to prevent postpartum haemorrhage: a network meta-analysis. Health technology assessment (Winchester, England) 23(9): 1–356 [PMC free article: PMC6421507] [PubMed: 30821683] |
- Study design not in PICO Health technology assessment |
| Ibrahim, ZM, Sayed Ahmed, WA, Abd El-Hamid, EM et al (2020) Carbetocin versus oxytocin for prevention of postpartum hemorrhage in hypertensive women undergoing elective cesarean section. Hypertension in pregnancy 39(3): 319–325 [PubMed: 32421401] |
- Duplicate Duplicate from IS search |
| Ibrahim, G. and Khalid, A. (2019) Is carbetocin as effective as oxytocin in preventing PPH in the third stage of labour in the emergency caesarean section?. Australian and New Zealand Journal of Obstetrics and Gynaecology 59(supplement1): 32 |
- Study design not in PICO Conference abstract |
| Islamy, N., Bernolian, N., Basir, F. et al (2018) The effect of different doses of intraumbilical oxytocin on the third stage of labor. International Journal of Gynecology and Obstetrics 143(supplement3): 288–289 |
- Non systemic administration Intraumbilical administration |
| Jaafar, J.D., Ismail, H., Ishak, N.A. et al (2019) Carbetocin versus syntometrine in the prevention of postpartum haemorrhage among women with risk factors following vaginal delivery. Medical Journal of Malaysia 74(supplement1): 24 |
- Study design not in PICO Conference abstract |
| Jaffer, Danish, Singh, Preet Mohinder, Aslam, Adam et al (2022) Preventing postpartum hemorrhage after cesarean delivery: a network meta-analysis of available pharmacologic agents. American journal of obstetrics and gynecology 226(3): 347–365 [PubMed: 34534498] |
- Study design not in PICO Conference abstract |
| Jiang, Danni, Yang, Yang, Zhang, Xinxin et al (2022) Continued versus discontinued oxytocin after the active phase of labor: An updated systematic review and meta-analysis. PloS one 17(5): e0267461 [PMC free article: PMC9060379] [PubMed: 35499990] |
- Intervention not in PICO Same drug intervention both arms only different routes of administration- continued versus discontinued oxytocin |
| Jin, Xin-Hang; Li, Dan; Li, Xia (2019) Carbetocin vs oxytocin for prevention of postpartum hemorrhage after vaginal delivery: A meta-analysis. Medicine 98(47): e17911 [PMC free article: PMC6882650] [PubMed: 31764790] |
- Systematic review Checked for eligible studies - eligible studies included. Ineligible studies either did not meet inclusion criteria or already included in Gallos 2018 |
| Kalafat, Erkan, Gokce, Ali, O'Brien, Pat et al (2021) Efficacy of carbetocin in the prevention of postpartum hemorrhage: a systematic review and Bayesian meta-analysis of randomized trials. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians 34(14): 2303–2316 [PubMed: 31537134] |
- Systematic review Checked for eligible studies - eligible studies included. Ineligible studies either did not meet inclusion criteria or already included in Gallos 2018 |
| Leas, B. and Umscheid, C. A. (2011) Active management and treatment of postpartum hemorrhage. | - Unavailable from IS search |
| Lewis, Lucy, Doherty, Dorota A, Conwell, Marion et al (2021) Spontaneous vaginal birth following induction with intravenous oxytocin: Three oxytocic regimes to minimise blood loss post birth. Women and birth : journal of the Australian College of Midwives 34(3): e322–e329 [PubMed: 32546384] |
- Intervention not in PICO Oxytocin used for induction of labour |
| Li, T, Wei, Q, Wu, L et al (2022) Multicenter, Randomized, Double-Blind, and Positive Drug-Controlled Clinical Trial on Prevention of Postpartum Hemorrhage after Vaginal Delivery with Ergometrine Maleate. Sichuan da xue xue bao. Yi xue ban [Journal of Sichuan University. Medical science edition] 53(2): 316–320 [PMC free article: PMC10409347] [PubMed: 35332736] |
- Unavailable from IS search Not available in English |
| Maged, AM, Fawzi, T, Shalaby, MA et al (2019) A randomized controlled trial of the safety and efficacy of preoperative rectal misoprostol for prevention of intraoperative and postoperative blood loss at elective cesarean delivery. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 147(1): 102–107 [PubMed: 31304593] |
- Intervention not in PICO Same drug intervention both arms - 400 μg rectal misoprostol at urinary catheter insertion plus 400 μg rectally after abdominal closure versus 800 μg of rectal misoprostol after abdominal closure |
| Maged, Ahmed M, Fawzi, Tarek, Shalaby, Mohamed A et al (2019) A randomized controlled trial of the safety and efficacy of preoperative rectal misoprostol for prevention of intraoperative and postoperative blood loss at elective cesarean delivery. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 147(1): 102–107 [PubMed: 31304593] |
- Duplicate Duplicate from IS search |
| Maged, Ahmed M, Wali, Ahmed A, Metwally, Ahmed A et al (2022) The efficacy of misoprostol in reducing intraoperative blood loss in women undergoing elective cesarean section. A systematic review and meta-analysis. The journal of obstetrics and gynaecology research [PubMed: 35661336] |
- Intervention not in PICO Same drug intervention both arms - preoperative versus postoperative misoprostol |
| Maged, Ahmed M, Waly, Mohamed, Fahmy, Radwa M et al (2020) Carbetocin versus rectal misoprostol for management of third stage of labor among women with low risk of postpartum hemorrhage. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 148(2): 238–242 [PubMed: 31736069] |
- Duplicate Duplicate from IS search |
| Mannaerts, D, Van der Veeken, L, Coppejans, H et al (2018) Adverse Effects of Carbetocin versus Oxytocin in the Prevention of Postpartum Haemorrhage after Caesarean Section: A Randomized Controlled Trial. Journal of pregnancy 2018: 1374150 [PMC free article: PMC5816867] [PubMed: 29484209] | - Included in Gallos 2018 |
| Mansouri, H.A. and Bahkali, D. (2018) A randomized controlled trial of intra-umbilical vein ergometrine as compared to intramuscular oxytocin for management of third stage of labor. Clinical and Experimental Obstetrics and Gynecology 45(4): 567–569 |
- Non systemic administration Intraumbilical administration |
| Masse, N.; Wong, C.; Dexter, F. (2022) A Randomized Controlled Trial to Assess Prophylactic Methylergonovine in Patients Undergoing an Intrapartum Cesarean Section. American Journal of Obstetrics and Gynecology 226(1supplement): 34 [PubMed: 35852267] |
- Duplicate Duplication from manual addition |
| Masuzawa, Yuko, Kataoka, Yaeko, Fujii, Kana et al (2018) Prophylactic management of postpartum haemorrhage in the third stage of labour: an overview of systematic reviews. Systematic reviews 7(1): 156 [PMC free article: PMC6180398] [PubMed: 30305154] |
- Systematic review Checked for eligible studies - eligible studies included. Ineligible studies either did not meet inclusion criteria or already included in Gallos 2018 |
| Mirteimouri, M, Pourali, L, Akhlaghi, F et al (2020) Effect of sublingual Misoprostol in combination with oxytocin in reducing blood loss during and after cesarean delivery: a randomized clinical trial. Tehran university medical journal 78(6): 357–365 |
- Unavailable from IS search Not available in English |
| Mohta, Medha, Chowdhury, Rohit B, Tyagi, Asha et al (2021) Efficacy of different infusion rates of oxytocin for maintaining uterine tone during elective caesarean section: A randomised double blind trial. Anaesthesia and intensive care 49(3): 183–189 [PubMed: 33934618] |
- Intervention not in PICO Same drug intervention both arms only different doses - Initial 1 IU bolus, then oxytocin infusion for four hours at 1.25 IU/hour versus 2.5 IU/hour versus 5.0 IU/hour |
| Mohta, Medha, Siddiqui, Sheeba, Chilkoti, Geetanjali T et al (2022) Oxytocin infusion rates for maintaining uterine tone during non-elective cesarean section in laboring patients: a randomized, controlled trial. Journal of anesthesia [PubMed: 35484429] |
- Intervention not in PICO Same drug intervention both arms only different doses - oxytocin infusions at rates of 2.5 IU/h versus 5 IU/h (Group 5) versus 10 IU/h (Group 10) following 3 IU slow bolus |
| Monte-Fenix, AP, Vera TR, GN (2011) Double-blind randomized controlled trial comparing the effect of carbetocin and oxytocin for the prevention of postpartum hemorrhage among high risk women following vaginal delivery. Philipp J Obstet Gynecol 35: 169–175 |
- Study design not in PICO Conference abstract |
| Moradan, S; Anaraki, RM; Mirmohammadkhani, M (2018) Prophylactic effect of misoprostol versus tranexamic acid in conjunction with oxytocin in reduction of post-partum hemorrhage after cesarean sectionin: a randomized clinical trial. Koomesh 20(4): 620–625 |
- Unavailable from IS search Not available in English |
| Muhammad, R., Isah, A., Agida, T. et al (2019) A prospective study to compare the effectiveness of adjunctive rectal misoprostol or oxytocin titration in the prevention of primary post-partum haemorrhage in at risk patients. African Health Sciences 19(1): 1517–1524 [PMC free article: PMC6531961] [PubMed: 31148979] |
- Study design not in PICO Case-control study |
| Naeem, M., Nawaz, F., Latif, M. et al (2021) Compare the sublingual and per rectal routes of misoprostol administration in third stage of labor in terms of average blood loss. Medical Forum Monthly 32(3): 105–108 |
- Intervention not in PICO Same drug intervention both arms only different routes of administration - 400 micro grams of sublingual misoprostol versus 400 micro grams of rectal misoprostol |
| Neyshabour University of Medical, Sciences (2022) The comparison of low dose with high dose of oxytocin in prevention of postpartum hemorrhage. |
- Unavailable from IS search Journal information unavailable so could no be found |
| Oladapo, O.T., Okusanya, B.O., Abalos, E. et al (2020) Intravenous versus intramuscular prophylactic oxytocin for reducing blood loss in the third stage of labour. Cochrane Database of Systematic Reviews 2020(12): cd009332 [PMC free article: PMC8236306] [PubMed: 33169839] |
- Intervention not in PICO Same drug intervention both arms only different routes of administration -IV versus IM oxytocin |
| Oladapo,, Olufemi T, Okusanya, Babasola O, Abalos, Edgardo et al (2020) Intravenous versus intramuscular prophylactic oxytocin for the third stage of labour. The Cochrane database of systematic reviews 11: cd009332 [PMC free article: PMC8236306] [PubMed: 33169839] |
- Duplicate Duplicate from IS search |
| Onwochei, D N, Van Ross,, J, Singh,, P M et al (2019) Carbetocin reduces the need for additional uterotonics in elective caesarean delivery: a systematic review, meta-analysis and trial sequential analysis of randomised controlled trials. International journal of obstetric anesthesia 40: 14–23 [PubMed: 31353178] |
- Systematic review Checked for eligible studies - eligible studies included. Ineligible studies either did not meet inclusion criteria or already included in Gallos 2018 |
| Onwochei, Desire N, Owolabi, Adetokunbo, Sinah, Preet Mohinder et al (2020) Carbetocin compared with oxytocin in non-elective Cesarean delivery: a systematic review, meta-analysis, and trial sequential analysis of randomized-controlled trials. Canadian journal of anaesthesia = Journal canadien d’anesthesie 67(11): 1524–1534 [PubMed: 32748189] |
- Systematic review Checked for eligible studies - eligible studies included. Ineligible studies either did not meet inclusion criteria or already included in Gallos 2018 |
| Peska, E., Balki, M., Maxwell, C. et al (2021) Oxytocin at elective caesarean delivery: a dose-finding study in women with obesity. Anaesthesia 76(7): 918–923 [PubMed: 33227150] |
- Study design not in PICO Dose finding study - no comparison/control group |
| Phung, Laura C, Farrington, Elise K, Connolly, Mairead et al (2021) Intravenous oxytocin dosing regimens for postpartum hemorrhage prevention following cesarean delivery: a systematic review and meta-analysis. American journal of obstetrics and gynecology 225(3): 250e1–250e38 [PubMed: 33957113] |
- Intervention not in PICO Same drug intervention both arms only different doses of IV oxytocin |
| Qian, XW, Drzymalski, DM, Lv, CC et al (2019) The ED50 and ED95 of oxytocin infusion rate for maintaining uterine tone during elective caesarean delivery: a dose-finding study. BMC preanancy and childbirth 20(1): 6 [PMC free article: PMC6937915] [PubMed: 31892352] |
- Intervention not in PICO Only one uterotonic - oxytocin infusion at a rate of 0, 1, 2, 3, 5, or 8 IU h- 1, to be given for a total of 1 hour |
| Salati, J.A., Leathersich, S.J., Williams, M.J. et al (2019) Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database of Systematic Reviews 2019(4): cd001808 [PMC free article: PMC6487388] [PubMed: 31032882] |
- Systematic review Checked for eligible studies - eligible studies included. Ineligible studies either did not meet inclusion criteria or already included in Gallos 2018 |
| Shah, M., Urooj, H., Shah, S. et al (2021) EFFICACY OF RECTAL MISOPROSTOL VS INTRAVENOUS OXYTOCIN IN PREVENTING POSTPARTUM HEMORRHAGE FOLLOWING ELECTIVE CAESAREAN SECTION. Journal of Postgraduate Medical Institute 35(3): 131–135 |
- Intervention not in PICO In one of the arms uterotonics were not administered as part of the third stage of labour |
| Slowiczek, L., Hein, D., Lozicki, A. et al (2018) Methylergonovine versus prostaglandins for postpartum hemorrhage: A systematic review and meta-analysis. Journal of the American Pharmacists Association 58(3): e83 |
- Study desian not in PICO Conference abstract |
| Somjit, Monsicha, Surojananon, Jaruta, Kongwattanakul, Kiattisak et al (2020) Comparison of Low Dose versus High Dose of Oxytocin for Initiating Uterine Contraction During Cesarean Delivery: A Randomized, Controlled, Non-Inferiority Trial. International journal of women's health 12: 667–673 [PMC free article: PMC7455765] [PubMed: 32904472] |
- Intervention not in PICO Same drug intervention both arms only different doses - intravenous injections of high-dose (10 IU) and low-dose (5 IU) oxytocin |
| Sun, Haiyan, Xu, Lei, Li, Yu et al (2022) Effectiveness and safety of carboxytocin versus oxytocin in preventing postpartum hemorrhage: A systematic review and meta-analysis. The journal of obstetrics and gynaecology research 48(4): 889–901 [PubMed: 35243717] |
- Systematic review Checked for eligible studies - eligible studies included. Ineligible studies either did not meet inclusion criteria or already included in Gallos 2018 |
| Suzhou Municipal, Hospital (2018) Therapeutic efficacy and safety of carbetocin on postpartum hemorrhage. |
- Unavailable from IS search Not available in English |
| Sweed, Mohamed, El-Said, Mourad, Abou-Gamrah, Amgad et al (2019) Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarian section: A randomized clinical trial. The journal of obstetrics and gynaecology research 45(3): 585–591 [PubMed: 30618101] |
- Intervention not in PICO Same drug intervention both arms only different doses - 200-, 400- or 600-mug misoprostol rectally |
| Tabl, S, Balki, M, Downey, K et al (2019) Uterotonics in elective caesarean delivery: a randomised non-inferiority study comparing carbetocin 20 mug and 100 mug. Anaesthesia 74(2): 190–196 [PubMed: 30506558] |
- Intervention not in PICO Only one uterotonic - intravenous carbetocin (20mug and 100mug) |
| Taheripanah, Robabeh, Shoman, Amal, Karimzadeh, Mohammad Ali et al (2018) Efficacy of oxytocin versus carbetocin in prevention of postpartum hemorrhage after cesarean section under general anesthesia: a prospective randomized clinical trial. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians 31(21): 2807–2812 [PubMed: 28707488] | - Included in Gallos 2018 |
| Tan, YQ, Liu, SJ, Cao, SY, Wang TT, CL (2018) Comparison of the effectiveness and safety of carbetocin and oxytocin in preventing postpartum hemorrhage after vaginal delivery: a meta-analysis. Chin J Evid Based Med 10: 1093–1100 |
- Unavailable from IS search Not available in Enalish |
| Torloni, Maria Regina, Siaulys, Monica, Riera, Rachel et al (2021) Timing of oxytocin administration to prevent post-partum hemorrhage in women delivered by cesarean section: A systematic review and metanalysis. PloS one 16(6): e0252491 [PMC free article: PMC8174699] [PubMed: 34081734] |
- Intervention not in PICO Same drug intervention both arms only different timing of oxytocin |
| Torloni, Maria Regina, Siaulys, Monica, Riera, Rachel et al (2021) Route of oxytocin administration for preventing blood loss at caesarean section: a systematic review with meta-analysis. BMJ open 11(9): e051793 [PMC free article: PMC8449971] [PubMed: 34531222] |
- Duplicate Duplication from IS search |
| University College of Mediacl Sciences and GTb, Hospital (2019) Comparison between mothers at high or low risk of uterine bleeding, with regards to the effective dose of oxytocin during cesarean surgery. |
- Unavailable from IS search Unavailable from IS search as no journal information |
| University of, Liverpool (2018) Carboprost vs Oxytocin as the First Line Treatment of Primary Postpartum Haemorrhage: A phase IV, double-blind, double-dummy, randomised controlled trial. |
- Unavailable from IS search Unavailable from IS search as no journal information |
| van der Nelson, H, O'Brien, S, Burnard, S et al (2021) Intramuscular oxytocin versus Syntometrine R versus carbetocin for prevention of primary postpartum haemorrhage after vaginal birth: a randomised double-blinded clinical trial of effectiveness, side effects and quality of life. BJOG : an international journal of obstetrics and gynaecology 128(7): 1236–1246 [PubMed: 33300296] |
- Duplicate Duplication from IS search |
| Vernekar, Sunil S, Goudar, Swati S, Metgud, Mrityunjay et al (2021) Effect of heat stable carbetocin vs oxytocin for preventing postpartum haemorrhage on post delivery hemoglobin-a randomized controlled trial. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians: 1–8 [PubMed: 34763599] |
- Study design not in PICO Sub analysis CHAMPION trial |
| Vice chancellor for Research, Tabriz University Of Medical Sciences (2021) Comparison of the effects of misoprostol and oxytocine on postpartum Hemorrhage. |
- Unavailable from IS search Journal information unavailable so could not be found |
| Voon, HY, Suharjono, HN, Shafie, AA et al (2018) Carbetocin versus oxytocin for the prevention of postpartum hemorrhage: A meta-analysis of randomized controlled trials in cesarean deliveries. Taiwanese journal of obstetrics & gynecology 57(3): 332–339 [PubMed: 29880160] |
- Systematic review Checked for eligible studies - eligible studies included. Ineligible studies either did not meet inclusion criteria or already included in Gallos 2018 |
| Wan, SQ, Pan, CY, Yin HZ, ZY (2018) Meta-analysis of the effectiveness and safety of carbetocin versus oxytocin in preventing post-cesarean hemorrhage. Drug Eval Res 8: 1504–1511 |
- Unavailable from IS search Not available in English |
| Wang, L.; Jiang, H.-M.; Yang, R.-R. (2020) Carboprost tromethamine prevents caesarean section-associated postpartum hemorrhage. Tropical Journal of Pharmaceutical Research 19(4): 899–904 |
- Non systemic administration Intrauterine administration |
| Wei, Lin Haiping; Sun, Xiaoli (2022) The Effect of Oxytocin plus Carboprost Methylate in Preventing Postpartum Hemorrhage in High-Risk Pregnancy and Its Effect on Blood Pressure. Evidence-based Complementary & Alternative Medicine (eCAM): 1–4 [PMC free article: PMC10307441] [PubMed: 37388077] |
- Intervention not in PICO Oxytocin versus Oxytocin plus carboprost methylate |
| Widmer, M., Piaggio, G., Nguyen, T.M.H. et al (2018) Heat-Stable Carbetocin Versus Oxytocin to Prevent Hemorrhage after Vaginal Birth. Obstetrical and Gynecological Survey 73(11): 613–614 [PubMed: 29949473] | - Included in Gallos 2018 |
| Widmer, M, Piaggio, G, Nguyen, TMH et al (2018) Heat-Stable Carbetocin Versus Oxytocin to Prevent Hemorrhage after Vaginal Birth. Obstetrical & gynecological survey 73(11): 613–614 [PubMed: 29949473] |
- Duplicate Duplication from IS search |
| Widmer, Mariana, Piaggio, Gilda, Nguyen, Thi M Het al (2018) Heat-Stable Carbetocin versus Oxytocin to Prevent Hemorrhage after Vaginal Birth. The New England journal of medicine 379(8): 743–752 [PubMed: 29949473] |
- Duplicate Duplication from IS search |
| Wu, Yu, Wang, Huan, Wu, Qi-Yan et al (2020) A meta-analysis of the effects of intramuscular and intravenous injection of oxytocin on the third stage of labor. Archives of gynecology and obstetrics 301(3): 643–653 [PubMed: 32124015] |
- Intervention not in PICO Same drug intervention both arms only different routes of administration- IV versus IM oxytocin |
| Xu, Renmei, Guo, Yongjie, Zhang, Qinggui et al (2022) Comparison of Clinical Efficacy and Safety between Misoprostol and Oxytocin in the Prevention of Postpartum Hemorrhage: A Meta-Analysis. Journal of healthcare engineering 2022:3254586 [PMC free article: PMC9017444] [PubMed: 35449871] |
- Systematic review Checked for eligible studies - eligible studies included. Ineligible studies either did not meet inclusion criteria or already included in Gallos 2018 |
| Yildirim, Dogukan and Ozyurek, Sefik Eser (2018) Intramuscular oxytocin administration before vs. after placental delivery for the prevention of postpartum hemorrhage: A randomized controlled prospective trial. European journal of obstetrics, gynecology, and reproductive biology 224: 47–51 [PubMed: 29533864] |
- Intervention not in PICO Same drug intervention both arms only different timings - 10 IU of oxytocin intramuscularly within the first minute following the delivery of the fetus versus 10 IU of intramuscular oxytocin immediately following placental delivery. |
| Zgaya, Rym, Ghadhab, Imen, Triki, Mohamed Amine et al (2020) Randomized controlled trial comparing 400mug sublingual misoprostol versus placebo for prevention of primary postpartum hemorrhage. The Pan African medical journal 36: 186 [PMC free article: PMC7467627] [PubMed: 32952830] |
- Duplicate Duplication from IS search |
| Zhou, Yuan-Hong, Xie, Yan, Luo, You-Zhen et al (2020) Intramuscular versus intravenous oxytocin for the third stage of labor after vaginal delivery to prevent postpartum hemorrhage: a meta-analysis of randomized controlled trials. European journal of obstetrics, gynecology, and reproductive biology 250: 265–271 [PubMed: 32439242] |
- Intervention not in PICO Same drug intervention both arms only different routes of administration - IV versus IM oxytocin |
Excluded economic studies
Table 48Excluded studies and reasons for their exclusion
| Study | Reason |
|---|---|
| Barrett, Jon; Ko,; Jeffery, William (2021) Cost Implications of Using Carbetocin Injection to Prevent Postpartum Hemorrhage in a Canadian Urban Hospital. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC [PubMed: 34656769] | - Cost analysis only |
| Lawrie, Theresa A., Rogozinska, Ewelina, Sobiesuo, Pauline et al (2019) A systematic review of the cost-effectiveness of uterotonic agents for the prevention of postpartum hemorrhage. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 146(1): 56–64 [PubMed: 31049950] | - Review |
| Luni, Yasmin, Borakati, Aditya, Matah, Arti et al (2017) A prospective cohort study evaluating the cost-effectiveness of carbetocin for prevention of postpartum haemorrhage in caesarean sections. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology 37(5): 601–604 [PubMed: 28317421] | - Cost analysis only |
| Mills, F., Siu, E., Poinas, A. C. et al (2014) A cost-minimization analysis of carbetocin for the prevention of postpartum hemorrhage in Canada. Value in Health 17(3): a161 | - Cost analysis only |
| Patel, B. and Haloob, R. (2014) Carbitocin: A cost-effective tool to save lives. BJOG: An International Journal of Obstetrics and Gynaecology 121(suppl2): 88–89 | - Conference abstract. |
| Pickering, Karen, Gallos, Ioannis D., Williams, Helen et al (2019) Uterotonic Drugs for the Prevention of Postpartum Haemorrhage: A Cost-Effectiveness Analysis. PharmacoEconomics -open 3(2): 163–176 [PMC free article: PMC6533349] [PubMed: 30506157] | - Duplicate analysis |
| Shaw, L., Morris, C., Baekgaard, E. et al (2013) Cost comparison of routine carbetocin use at caesarean section. BJOG: An International Journal of Obstetrics and Gynaecology 120(suppl1): 119–120 | - Conference abstract. |
| van der Nelson, Helen A., Draycott, Tim, Siassakos, Dimitrios et al (2017) Carbetocin versus oxytocin for prevention of post-partum haemorrhage at caesarean section in the United Kingdom: An economic impact analysis. European journal of obstetrics, gynecology, and reproductive biology 210: 286–291 [PubMed: 28088109] | - Cost consequence analysis. In addition it was considered that this study could not helpfully inform recommendations as there was more recent research and a de Novo model produced for the guideline which included a broader range of uterotonic comparators as well as more recent clinical evidence which was synthesised through a network meta-analysis |
| Wohling, J., Edge, N., Pena, Leal D. et al (2018) Cost comparison of carbetocin compared to oxytocin as primary postpartum haemorrhage (PPH) prophylaxis at caesarean section. Australian and New Zealand Journal of Obstetrics and Gynaecology 58(supplement1): 88 | - Conference abstract. |
Appendix K. Research recommendations – full details
Research recommendations for review question: What is the effectiveness of uterotonics for the prevention of postpartum haemorrhage?
No research recommendations were made for this review question.
Appendix L. Network meta-analysis methods
Download PDF (618K)
Appendix M. Model fit results
Download PDF (205K)
Appendix N. Inconsistency checks
Download PDF (1.3M)
Final version
Evidence reviews underpinning recommendations 1.10.9 to 1.10.11 and 1.10.13 in the NICE guideline
These evidence reviews were developed by NICE
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Uterotonic drugs to prevent postpartum haemorrhage: a network meta-analysis.[Health Technol Assess. 2019]Uterotonic drugs to prevent postpartum haemorrhage: a network meta-analysis.Gallos I, Williams H, Price M, Pickering K, Merriel A, Tobias A, Lissauer D, Gee H, Tunçalp Ö, Gyte G, et al. Health Technol Assess. 2019 Feb; 23(9):1-356.
- Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis.[Cochrane Database Syst Rev. 2018]Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis.Gallos ID, Papadopoulou A, Man R, Athanasopoulos N, Tobias A, Price MJ, Williams MJ, Diaz V, Pasquale J, Chamillard M, et al. Cochrane Database Syst Rev. 2018 Dec 19; 12(12):CD011689. Epub 2018 Dec 19.
- Review Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage.[Cochrane Database Syst Rev. 2013]Review Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage.Westhoff G, Cotter AM, Tolosa JE. Cochrane Database Syst Rev. 2013 Oct 30; (10):CD001808. Epub 2013 Oct 30.
- Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage.[Cochrane Database Syst Rev. 2019]Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage.Salati JA, Leathersich SJ, Williams MJ, Cuthbert A, Tolosa JE. Cochrane Database Syst Rev. 2019 Apr 29; 4(4):CD001808. Epub 2019 Apr 29.
- Review Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis.[Cochrane Database Syst Rev. 2018]Review Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis.Gallos ID, Williams HM, Price MJ, Merriel A, Gee H, Lissauer D, Moorthy V, Tobias A, Deeks JJ, Widmer M, et al. Cochrane Database Syst Rev. 2018 Apr 25; 4(4):CD011689. Epub 2018 Apr 25.
- Evidence reviews for uterotonics for the prevention of postpartum haemorrhageEvidence reviews for uterotonics for the prevention of postpartum haemorrhage
Your browsing activity is empty.
Activity recording is turned off.
See more...