About GTR®
Participation in the GTR is voluntary. There is no charge to submit data or to use the website.
This document contains information about the definition and regulation of tests, the types of tests contained in the Genetic Testing Registry (GTR) and how to recognize them, and how search results are ranked. The table of contents expedites navigation to the section of interest.
Selected references about the GTR are cited at the end of this document.
Definition of a Genetic Test in the GTR
A genetic test involves an analysis of human chromosomes, deoxyribonucleic acid, ribonucleic acid, genes, and/or gene products (e.g., enzymes and other types of proteins), which is predominately used to detect heritable or somatic mutations, genotypes, or phenotypes related to disease and health.
[Definition is adapted from the April 2008 Report of the Secretary's Advisory Committee on Genetics, Health, and Society (SACGHS) on the U.S. System of Oversight of Genetic Testing: A Response to the Charge of the Secretary of Health and Human Services (page 17).]
Clinical and Research Tests
GTR currently contains information about genetic and microbe tests intended for clinical use and also accepts registrations for genetic and microbe research tests.
Definition of a Research Test
For the purposes of the GTR, a research test is defined as a test that is performed for the purpose of contributing to generalizable knowledge or for a laboratory to generate data in order to make technical improvements to a test.
[Definition is based on the definition of research used in Title 45 CFR part 46 of Health and Human Services regulations (46.102(d)], which codifies the U.S. Federal Policy for the Protection of Human Subjects, or the Common Rule. The definition also aligns with the medical research definition used in the international Declaration of Helsinki (#7).]
Current Test Content in the GTR
The Genetic Testing Registry contains information about clinical and research tests for Mendelian disorders and drug responses as well as somatic/cancer variation. GTR includes includes multigenic, array-based, biochemical, cytogenetic, and molecular tests. Molecular and serologic clinical and research tests for microorganisms - bacteria, viruses, and parasites - that play a role in human health and infectious disease are also within scope for GTR; examples, nucleic acid amplification and serologic tests for SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), the virus which causes COVID-19, real-time multiplex RT-PCR tests for norovirus, a gastrointestinal pathogen, and tests for the human microbiome.
GTR content comes from these information sources:
- Test providers who register in GTR by submitting laboratory and test data, and
- Automatically provided data supplemented from NCBI’s databases
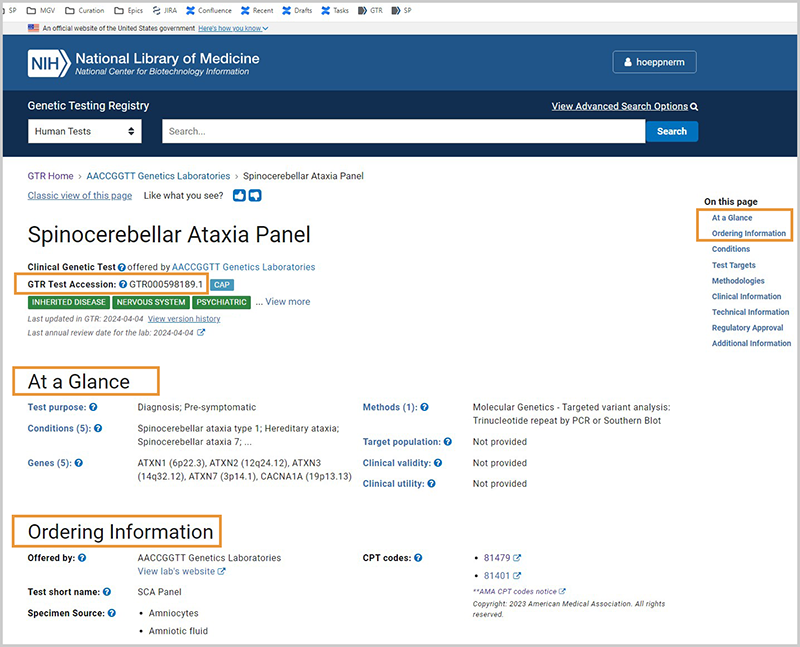
Appearance of registered GTR tests
Registered tests have:
- A unique GTR accession and version number located below the test name
- Test names that are specified by the submitting laboratory
- Content for the test is in several sections on the page, that can be accessed using the navigation tool
To view the information content offered by the GTR, explore a registered GTR test and the corresponding laboratory record.
Send-out tests
GTR does not include tests that are performed entirely at an outside lab/facility (send-outs).
Regulatory Information in GTR
In the U.S., clinical tests may be provided only by laboratories certified by the Centers for Medicare & Medicaid Services (CMS) through the Clinical Laboratory Improvement Amendments of 1988 (CLIA). The GTR includes information about CLIA status if provided by the laboratory, including CLIA certification numbers and expiration dates.
Not all tests entered into the GTR have been cleared or approved by the U.S. Food and Drug Administration (FDA). GTR includes information about FDA test review where available. This information is provided by the submitter and is not independently reviewed by NIH or FDA. FDA has generally exercised enforcement discretion over a class of in vitro diagnostics termed Laboratory Developed Tests (LDTs). More information about FDA oversight of LDTs can be found here.
Community standards for Good Laboratory Practices are available for review for biochemical genetic testing and newborn screening and molecular genetic testing.
Ranking of Search Results
Search results are primarily determined by the search term chosen by the user and the filtering strategy. Weighting is applied to present records to the user that contain more detailed information among initial results.
Searches that produce a list of results are ordered according to the following factors:
-
Relevance sorting evaluates the presence and frequency of the search term within records. The sort order is ranked according to relevance. A limit is set for how much a search term can increase relevance.
-
Tests containing more completed recommended fields have a higher sort order than others. For example, registered tests with completed fields for Clinical utility, Clinical validity and Target population are weighted more heavily than tests without this information.
- The laboratory comparison page is alphabetical by institution. If there is no institution, the list is alphabetical by laboratory name.
Selected References about the GTR
- The NIH genetic testing registry: a new, centralized database of genetic tests to enable access to comprehensive information and improve transparency
Wendy S. Rubinstein; Donna R. Maglott; Jennifer M. Lee; Brandi L. Kattman; Adriana J. Malheiro; Michael Ovetsky; Vichet Hem; Viatcheslav Gorelenkov; Guangfeng Song; Craig Wallin; Nora Husain; Shanmuga Chitipiralla; Kenneth S. Katz; Douglas Hoffman; Wonhee Jang; Mark Johnson; Fedor Karmanov; Alexander Ukrainchik; Mikhail Denisenko; Cathy Fomous; Kathy Hudson; James M. Ostell
Nucleic Acids Research 2013; 41 (D1): D925-D935. doi: 10.1093/nar/gks1173
Abstract Full Text PDF
GTR is a Registered Trademark
GTR is Registered, U.S. Patent and Trademark Office under Registration Number 4,302,210.
