The GTR Submission Process: Annual Review
OMB NO: 0925-0651
EXPIRATION DATE: 01/31/2025
This document explains GTR's Annual Review policy. It provides information to help submitters successfully complete the Annual Review.
Purpose of the Annual Review in GTR
The overall purpose of the Annual review is to meet the mutually beneficial goal of having accurate and complete data in the registry. During the Annual Review, you can:
- Review and confirm the accuracy of your laboratory and test information on at least an annual basis as agreed upon when accepting the Code of Conduct.
- Ensure all registered tests are still offered by the laboratory.
- Provide more complete information on your laboratory and tests, if desired.
Thank you for your active participation in GTR.
Policies
Current and updated policies are as follows:
- You can start the annual review for your laboratory at any time. However, you will only need to take action as the due date for your annual review approaches. Note that each annual review form that is initiated gets a unique submission identifier.
- You may log into the Submission Portal at any time to update laboratory information and to add, update, and delete tests.
- If the annual review has been started but not completed for over 90 days, the annual review record (form) will be automatically deleted (Effective November 1, 2024).
- If the annual review has not been performed for more than one year past the due date, then laboratory and test records will be marked ‘Past due’ for the annual review on GTR's public website. Completing the annual review resets the date and removes the ‘Past due’ mark on all records.
-
GTR will send automatic reminders to submitters about the annual review at the following times:
- Around the due date (1 month before the due date, 1 week before the due date, on the due date)
- One year past the due date (i.e., no annual review for 2 years).
- One and a half years past the due date (i.e., no annual review for 2 years, 6 months). At this time, GTR staff will reach out to the laboratory to ensure that notifications are reaching the laboratory and to provide assistance (Effective date November 1, 2024).
-
GTR will remove records if the annual review has not been completed for more than three years. At this time, if records have been updated but the annual review has not been completed, GTR staff will reach out to submitters to offer assistance (Effective date November 1, 2024).
How to access the Annual Review
You can start the Annual Review from the home page for your lab in GTR Submission Portal. In the section 'Annual Review', click on the link 'Perform annual review' as shown below:
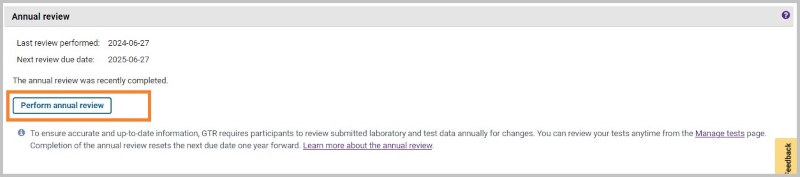
This will open the page for the Annual Review (updated annual review form to be released in July 2024)
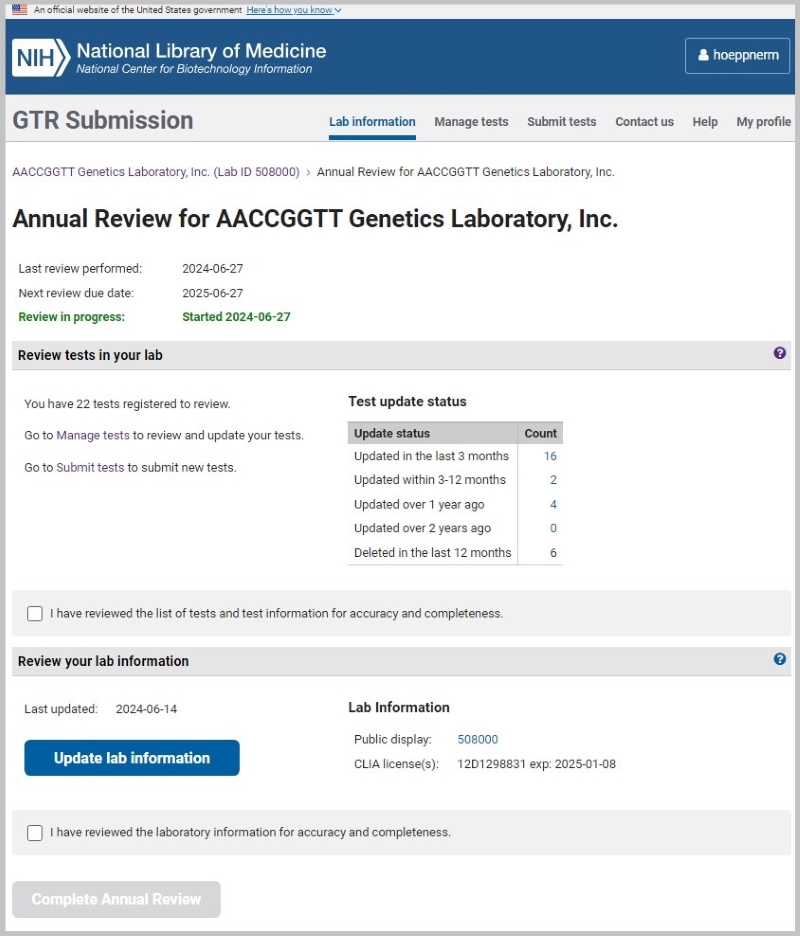
Review your lab information
Make any necessary updates to your laboratory information. Click on ‘Update lab information’. You can update personnel, contacts, license expiration dates, website, and other lab information.
Review tests in your lab
- Click on 'Manage Tests' to navigate to the test management page where you can copy, update, or delete tests, perform bulk updates using the submission wizard, and download select tests; see Manage tests.
- Click on 'Submit tests' to go to the submission page where you can add new tests using a the submission wizard, upload spreadsheet submission files, and access data about your API submissions; see Submit tests. From the submission home page, click the 'Download tests' button to download GTR clinical test data. You will receive an email with the data in the spreadsheet submission template. You can use the spreadsheet files for submission.
-
The annual review form will show when your tests were last updated or deleted. You can view the list of tests in each category in the 'Manage tests' page.
- Updated in the last 3 months
- Updated within 3-12 months
- Updated over 1 year ago
- Updated over 2 years ago
- Deleted in the last 12 months
Complete your annual review
Once you have reviewed and updated your laboratory and test information, check the following boxes:
- I have reviewed the list of tests and test information for accuracy and completeness.
- I have reviewed the laboratory information for accuracy and completeness.
Click 'Complete Annual Review'.
You will get a confirmation email.
Tips on finalizing the Annual Review
- Making updates to your lab and test records as part of the Annual Review will cause you to navigate away from the Annual Review page. Please remember to return to the Annual Review window and click the button ‘Complete Annual Review’ to finalize your work.
- Confirmation emails about your laboratory and test submissions are not confirmations about completion of your Annual Review.
- When updating your lab and test records, be sure to click the 'Submit' button.
Q & A about Annual Review procedures
Q. Who can perform the Annual Review?
A. For a particular laboratory, any personnel with submission privileges for that laboratory may perform the Annual Review.
Q. What are the minimal requirements for completing an Annual Review?
A. The only strict requirement is that you must complete the Annual Review form, confirming that your laboratory and test data is up to date. Please keep in mind that by accepting the Code of Conduct you agreed ‘to uphold the integrity of the GTR through the submission of information that is accurate and not misleading’ and ‘to assure the accuracy of the data at the time of submission and to review and, if necessary, update the submitted information at least once a year’.
Q. What other steps are recommended, but not required to complete the Annual Review?
A. Review your Laboratory record e.g., CLIA expiration date and staff list and update as needed. We recommend that you alert us if any staff members with submission privileges have left your lab so that we can remove their access to your submission data.
Q. How will my laboratory’s due date be reset following completion of the first Annual Review?
A. Completing the first Annual Review sets the next due date one year forward.
GTR Support
For help with your submission, contact GTR staff.
