NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Review question: What interventions for chronic hypertension are effective at improving outcomes for women and infants?
Introduction
Chronic hypertension in pregnancy is hypertension present at the booking visit or before 20 weeks, or if the woman is already taking antihypertensive medication when presenting to maternity services. It can be primary or secondary in aetiology. Its pathophysiology is likely to be different from gestational hypertension, and intervention in chronic hypertension which are successful in reducing complications in the mother and baby may be different from those interventions which improve outcomes in gestational hypertension.
This review will look at the evidence for interventions in chronic hypertension in pregnancy to determine which improve outcomes in the woman and her baby.
Summary of the protocol
See Table 1 for a summary of the population, intervention, comparison and outcome (PICO) characteristics of this review.
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual 2014. Methods specific to this review question are described in the review protocol in appendix A.
Declaration of interests were recorded according to NICE’s 2018 conflicts of interest policy (see Register of interests).
Clinical evidence
Included studies
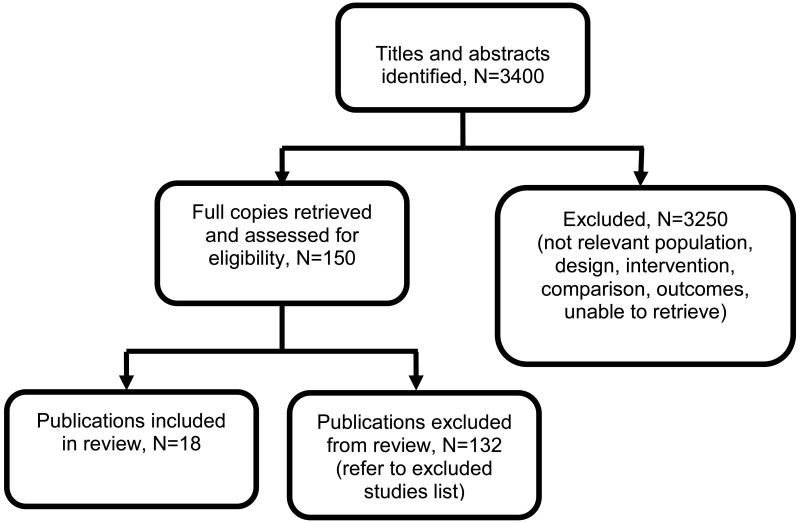
Eighteen articles from 15 randomised controlled trials (RCTs) and 2 individual participant data (IPD) meta-analyses of RCT data have been included in this review (N=5377) (Askie 2007, Atallah 1996, Butters 1990, Cockburn 1982, Hamed 2014, Kasawara 2013, Magee 2015, Moore 1982, Moore 2015, Parazzini 1993, Poon 2017, Redman 1976, Sibai 1990, van Vliet 2017, Vigil-de Gracia 2014, Viinikka 1993, Webster 2017, Weitz 1987).
Summary estimates were reported in the two IPD meta-analyses. However, as the articles did not report the specific data from each of the original studies it was not possible to pool the estimates from the IPD meta-analyses with additional studies. Instead, data from the IPD meta-analyses are presented separately to that from the additional RCTs identified in this review.
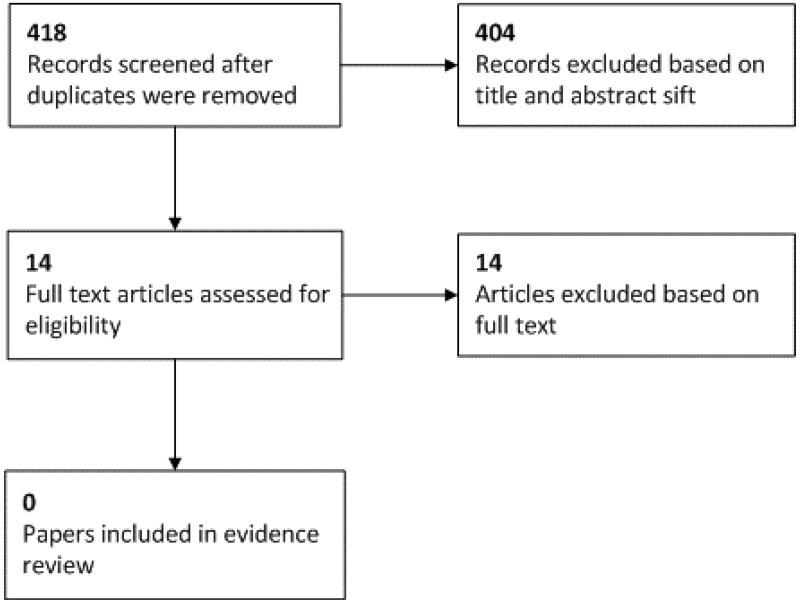
See the literature search strategy in appendix B and study selection flow chart in appendix C.
Excluded studies
Studies not included in this review, with reasons for their exclusion, are provided in appendix K.
Summary of clinical studies included in the evidence review
Table 2 provides a brief summary of the included studies.
See appendix D for clinical evidence tables.
Quality assessment of clinical outcomes included in the evidence review
See appendix F for full GRADE tables.
Economic evidence
No economic evidence on the cost effectiveness interventions for chronic hypertension was identified by the systematic search of the economic literature undertaken for this guideline. Economic modelling was not undertaken for this question because other topics were agreed as higher priorities for economic evaluation.
Evidence statements
Comparison 1. Induction of labour versus expectant management
Outcomes for babies
Critical outcomes
Perinatal mortality
- One randomised controlled trial (n=76) provided very low quality evidence to show that there were no clinically important differences in perinatal mortality between those who received induction of labour or expectant management.
Important outcomes
Birth weight
- One randomised controlled trial (n=76) provided low quality evidence to show a clinically important decrease in the weight of babies born of women who received induction of labour compared to those of women who received expectant management.
Gestational age at birth
- One randomised controlled trial (n=76) provided very low quality evidence to show a clinically important decrease in the gestational age at birth for babies born of women who received induction of labour as compared to those of women who received expectant management.
Preterm birth (number of weeks not reported)
- One randomised controlled trial (n=76) provided very low quality evidence to show no clinically important differences in the number of preterm births between those who received induction of labour or expectant management.
Admission to neonatal unit
- One randomised controlled trial (n=76) provided very low quality evidence to show a clinically important increase in the number of babies admitted to a neonatal unit between women who received induction of labour as compared to expectant management.
Outcomes for women
Critical outcomes
Severe hypertension
- One randomised controlled trial (n=76) provided very low quality evidence to show no clinically important difference in the occurrence of severe hypertension between those who received induction of labour or expectant management.
Important outcomes
Superimposed pre-eclampsia
- One randomised controlled trial (n=76) provided very low quality evidence to show no clinically important difference in the incidence of superimposed pre-eclampsia between those who received induction of labour or expectant management.
Placental abruption
- One randomised controlled trial (n=76) provided very low quality evidence to show no clinically important difference in the occurrence of placental abruption between those who received induction of labour or expectant management.
Comparison 2. Exercise versus no intervention
Outcomes for babies
Important outcomes
Birth weight
- One randomised controlled trial (n=109) provided very low quality evidence to show that there were no clinically important differences in birth weight between the babies born of mothers who exercised and those who did not exercise, for weights of <2500g or 2500-3999g. There may be a clinically important reduction in the number of babies born weighing ≥4000g for those who exercise, but there was some uncertainty around the effect (RR 0.43, 95% CI 0.16-1.16).
Admission to neonatal unit
- One randomised controlled trial (n=109) provided very low quality evidence to show no clinically important difference in neonatal unit admission between babies born of mothers who exercised and those who did not exercise.
Outcomes for women
Important outcomes
Mode of birth (caesarean section)
- One randomised controlled trial (n=109) provided very low quality evidence to show no clinically important differences in mode of birth (caesarean section) between women who exercised and those who did not exercise.
Comparison 3. Less-tight versus tight control of blood pressure
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=981) provided very low quality evidence to show no clinically important difference in the occurrence of stillbirth between those who received less-tight or tight control of blood pressure.
Neonatal death
- One randomised controlled trial (n=981) provided very low quality evidence to show no clinically important difference in the occurrence of neonatal death between those who received less-tight or tight control of blood pressure.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=727) provided low quality evidence to show a clinically important decrease in the number of babies born small-for-gestational age for women who received less-tight control of blood pressure, as compared to women who received tight control of blood pressure.
Important outcomes
Birth weight
- One randomised controlled trial (n=981) provided low quality evidence to show no clinically important difference in the birth weight of babies born to women who received less-tight or tight control of blood pressure.
Gestational age at birth
- One randomised controlled trial (n=981) provided low quality evidence to show no clinically important difference in the gestational age at birth for babies born to women who received less-tight or tight control of blood pressure.
Admission to neonatal unit
- One randomised controlled trial (n=959) provided low quality evidence to show no clinically important difference in neonatal unit admissions for babies born to women who received less-tight or tight control of blood pressure.
Outcomes for women
Critical outcomes
Severe hypertension
- One randomised controlled trial (n=732) provided moderate quality evidence to show that less-tight blood pressure control resulted in a clinically important increase in the number of women experiencing severe hypertension, as compared to those with tight control.
Important outcomes
Haemolysis, elevated liver enzymes, low platelets (HELLP)
- One randomised controlled trial (n=981) provided very low quality evidence to show that there may be a clinically important increase in the occurrence of HELLP for those receiving less-tight control, as compared to those receiving tight control, but there was some uncertainty around the estimate (RR 4.45, 95% CI 0.97 to 20.51).
Placental abruption
- One randomised controlled trial (n=981) provided very low quality evidence to show no clinically important difference in the occurrence of placental abruption between those who received less-tight or tight control of blood pressure.
Pre-eclampsia
- One randomised controlled trial (n=731) provided low quality evidence to show no clinically important difference in the occurrence of pre-eclampsia between those who received less-tight or tight control of blood pressure.
Onset of labour (spontaneous onset)
- One randomised controlled trial (n=981) provided very low quality evidence to show no clinically important difference in the number of women experiencing spontaneous onset of labour for those who received less-tight or tight control of blood pressure.
Onset of labour (induced onset)
- One randomised controlled trial (n=981) provided low quality evidence to show no clinically important difference in the number of women experiencing induction of labour for those who received less-tight or tight control of blood pressure.
Onset of labour (elective caesarean)
- One randomised controlled trial (n=981) provided low quality evidence to show no clinically important difference in the occurrence of elective caesarean section for those who received less-tight or tight control of blood pressure.
Mode of birth (caesarean section)
- One randomised controlled trial (n=981) provided low quality evidence to show no clinically important difference in the rate of caesarean section for those who received less-tight or tight control of blood pressure.
Comparison 4. Atenolol versus placebo
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=29) provided very low quality evidence to show no clinically important difference in the occurrence of stillbirth between those who received placebo or atenolol.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=29) provided low quality evidence to show a clinically important increase in the number of babies born small-for-gestational age for those who received atenolol, as compared to those who received placebo.
Important outcomes
Birth weight
- One randomised controlled trial (n=29) provided very low quality evidence to show a clinically important decrease in birth weight for babies of women who received atenolol, as compared to those who received placebo.
Gestational age at birth
- One randomised controlled trial (n=29) provided very low quality evidence to show a mean gestational age of 39.5 weeks for infants born to women taing placebo, and a mean gestational age of 38.5 weeks for infants born to women taking atenolol.
Outcomes for women
Critical outcomes
Blood pressure control
- One randomised controlled trial (n=29) provided very low quality evidence to show a clinically important decrease in diastolic blood pressure for those who received atenolol, as compared to those who received placebo. However, this same study provided very low quality evidence to show that there was no clinically important difference in the systolic blood pressure measurements between those who received atenolol and placebo.
Comparison 5. Labetalol versus no intervention
Outcomes for babies
Critical outcomes
Perinatal death up to 7 days
- One randomised controlled trial (n=176) provided very low quality evidence to show no clinically important difference in perinatal deaths between those who received labetalol or no intervention.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=176) provided very low quality evidence to show that there was no clinically important difference in the number of babies born small-for-gestational age between those who received labetalol or no intervention.
Important outcomes
Preterm birth (<37 weeks)
- One randomised controlled trial (n=176) provided very low quality evidence to show that there was no clinically important difference in preterm birth (<37 weeks) for those who received labetalol or no intervention.
Outcomes for women
Important outcomes
Superimposed pre-eclampsia
- One randomised controlled trial (n=176) provided very low quality evidence to show that there was no clinically important difference in the number of women developing superimposed pre-eclampsia between those who received labetalol or no intervention.
Placental abruption
- One randomised controlled trial (n=176) provided very low quality evidence to show that there was no clinically important difference in the occurrence of placental abruption between those who received labetalol or no intervention.
Mode of birth (caesarean section)
- One randomised controlled trial (n=176) provided very low quality evidence to show that there was no clinically important difference in the number of women undergoing caesarean section between those who received labetalol or no intervention.
Comparison 6. Labetalol versus nifedipine
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the occurrence of stillbirth between those who received labetalol or nifedipine.
Neonatal death up to 7 days
- One randomised controlled trial (n=112) provided moderate quality evidence to show that no neonatal deaths occurred in those who received labetalol or nifedipine.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the number of babies born small-for-gestational age between those who received labetalol or nifedipine.
Important outcomes
Birth weight
- One randomised controlled trial (n=112) provided low quality evidence to show no clinically important difference in the birth weight of babies born to women who received labetalol or nifedipine.
Preterm birth (<37 weeks)
- One randomised controlled trial (n=112) provided low quality evidence to show no clinically important difference in the occurrence of preterm birth (<37 weeks) between those who received labetalol or nifedipine.
Preterm birth (<34 weeks)
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the occurrence of preterm birth (<34 weeks) between those who received labetalol or nifedipine.
Admission to neonatal unit
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the number of babies requiring neonatal unit admission between women who received labetalol or nifedipine.
Gestational age at birth
- One randomised controlled trial (n=112) provided moderate quality evidence to show a clinically important increase in the gestational age at birth for the babies of women who received labetalol compared to women who received nifedipine.
Outcomes for women
Important outcomes
Mode of birth (caesarean section)
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the number of women giving birth by caesarean section between those who received labetalol or nifedipine.
Superimposed pre-eclampsia
- One randomised controlled trial (n=112) provided low quality evidence to show no clinically important difference in the number of women developing superimposed pre-eclampsia between those who received labetalol or nifedipine.
Superimposed pre-eclampsia <34 weeks
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the occurrence of early onset superimposed pre-eclampsia (< 34 weeks) between those who received labetalol or nifedipine.
Eclampsia
- One randomised controlled trial (n=112) provided moderate quality evidence to show no occurrence of eclampsia in women who received labetalol or nifedipine.
Maternal death
- One randomised controlled trial (n=112) provided moderate quality evidence to show that no maternal deaths occurred in those who received labetalol or nifedipine.
Comparison 7. Labetalol versus methyldopa
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=72) provided very low quality evidence to show that no stillbirths occurred in those who received labetalol or methyldopa.
Neonatal death up to 7 days
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no clinically important difference in neonatal death between those who received labetalol or methyldopa.
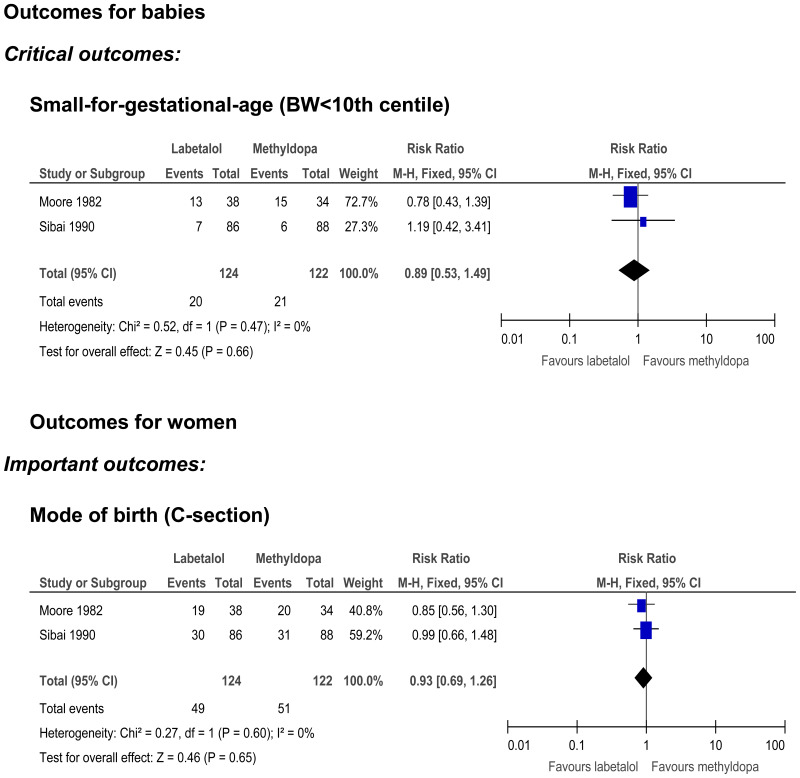
Small-for-gestational age
- Two randomised controlled trials (n=246) provided very low quality evidence to show no clinically important difference in the number of babies born small-for-gestational age between women who received labetalol or methyldopa.
Important outcomes
Birth weight
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no clinically important difference in infant birth weight between women who received labetalol or methyldopa.
Gestational age at birth
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no clinically important difference in the gestational age at birth for babies born to women who received labetalol or methyldopa.
Admission to neonatal unit
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no difference in the rate of admission to a neonatal unit for babies of women who received labetalol or methyldopa.
Outcomes for women
Critical outcomes
Blood pressure control
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no clinically important difference in the systolic or diastolic blood pressure measurements between those who received labetalol or methyldopa.
Important outcomes
Onset of labour (induction)
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no clinically important difference in the number of women undergoing induction of labour between those who received labetalol or methyldopa.
Mode of birth (caesarean section)
- Two randomised controlled trials (n=246) provided very low quality evidence to show that there was no clinically important difference in the incidence of caesarean section between those who received labetalol or methyldopa.
Comparison 8. Methyldopa versus placebo
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=25) provided moderate quality evidence to show that no stillbirths occurred in those who received methyldopa or placebo.
Neonatal death
- One randomised controlled trial (n=25) provided moderate quality evidence to show that no neonatal deaths occurred in those who received methyldopa or placebo.
Important outcomes
Gestational age at birth
- One randomised controlled trial (n=25) provided moderate quality evidence to show a clinically important increase in the gestational age at birth for infants of women who received methyldopa compared to those of women who received placebo.
Outcomes for women
Important outcomes
Superimposed pre-eclampsia
- One randomised controlled trial (n=25) provided very low quality evidence to show no clinically important difference in the occurrence of superimposed pre-eclampsia between those who received methyldopa or placebo.
Comparison 9. Methyldopa versus no intervention
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=190) provided very low quality evidence to show a clinically important reduction in stillbirths for those who received methyldopa, compared to no intervention.
Perinatal death
- One randomised controlled trial (n=178) provided very low quality evidence to show no clinically important difference in perinatal death rates between those who received methyldopa or no intervention.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=178) provided very low quality evidence to show that there was no clinically important difference in the number of babies born small-for-gestational age between women who received methyldopa or no intervention.
Important outcomes
Birth weight
- One randomised controlled trial (n=190) provided low quality evidence to show no clinically important difference in the birth weight of babies born to women who received methyldopa or no intervention.
Gestational age at birth
- One randomised controlled trial (n=204) provided low quality evidence to show no clinically important difference in the gestational age of babies born to women who received methyldopa or no intervention.
Preterm birth (<37 weeks)
- One randomised controlled trial (n=178) provided very low quality evidence to show that there was no clinically important difference in preterm births (<37 weeks) between those who received methyldopa or no intervention.
Neurodevelopmental outcomes at ≥ 18 months: impaired vision at 7.5 years old
- One randomised controlled trial (n=190) provided very low quality evidence to show that there may be a clinically important decrease in the number of children with impaired vision at 7.5 years old for those who received methyldopa, as compared to placebo, but there was some uncertainty around the effect (RR 0.47, 95% CI 0.20 to 1.11).
Neurodevelopmental outcomes at ≥ 18 months: impaired hearing at 7.5 years old
- One randomised controlled trial (n=188) provided very low quality evidence to show that there was no clinically important difference in impaired hearing at 7.5 years follow-up between children born to women who received methyldopa or no intervention
Outcomes for women
Important outcomes
Superimposed pre-eclampsia
- One randomised controlled trial (n=178) provided very low quality evidence to show that there was no clinically important difference in the development of superimposed pre-eclampsia between those who received methyldopa or no intervention.
Placental abruption
- One randomised controlled trial (n=178) provided very low quality evidence to show that there was no clinically important difference in the incidence of placental abruption between those who received methyldopa or no intervention
Mode of birth (caesarean section)
- One randomised controlled trial (n=178) provided very low quality evidence to show that there was no clinically important difference in the number of women giving birth by caesarean section between those who received methyldopa or no intervention
Comparison 10. Amlodipine versus aspirin
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the occurrence of stillbirth between those who received amlodipine or aspirin.
Neonatal death
- One randomised controlled trial (n=39) provided moderate quality evidence to show that no neonatal deaths occurred in those who received amlodipine or aspirin.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the number of babies born small-for-gestational age between those who received amlodipine or aspirin.
Important outcomes
Birth weight
- One randomised controlled trial (n=39) provided low quality evidence to show no clinically important difference in the birth weight of infants born to women who received amlodipine or aspirin.
Preterm birth (weeks not specified)
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the occurrence of preterm birth between those who received amlodipine or aspirin.
Outcomes for women
Critical outcomes
Severe hypertension
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the incidence of severe hypertension between those who received amlodipine or aspirin.
Important outcomes
Placental abruption
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the occurrence of placental abruption between those who received amlodipine or aspirin.
Mode of birth (caesarean section)
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the number of women giving birth by caesarean section between those who received amlodipine or aspirin.
Comparison 11. Aspirin versus no intervention
Outcomes for babies
Critical outcomes
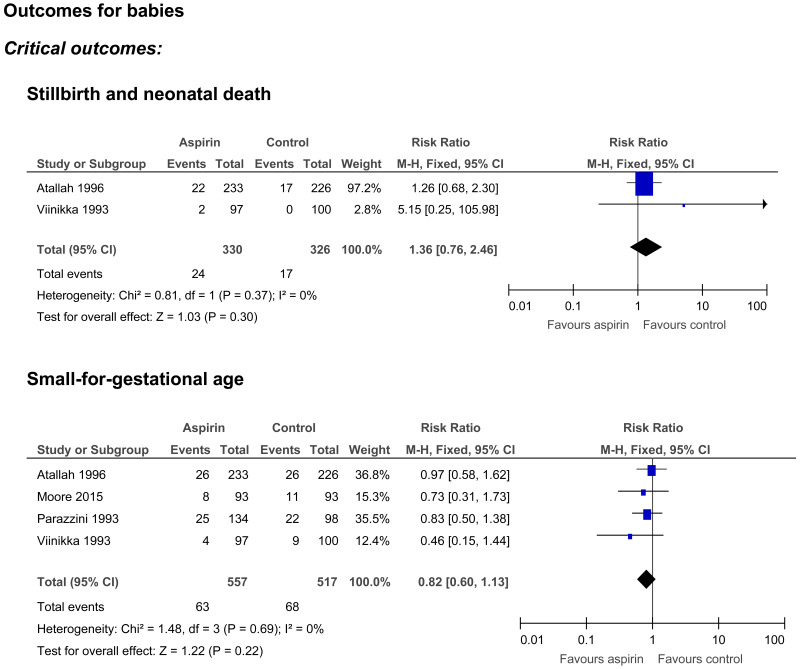
Stillbirth and neonatal death
- Two randomised controlled trials (n=656) provided very low quality evidence to show no clinically important difference in the occurrence of stillbirth and neonatal death between those who received aspirin or no intervention.
Small for gestational age
- Four randomised controlled trials (n=1074) provided very low quality evidence to show no clinically important difference in the number of babies born small-for-gestational age between women who received aspirin or no intervention.
Important outcomes
Birth weight
- One randomised controlled trial (n=197) provided low quality evidence to show no clinically important difference in the birth weight of babies born to women who received aspirin or no intervention.
Gestational age
- One randomised controlled trial (n=197) provided moderate quality evidence to show that there was no clinically important difference in the gestational age at birth of babies born to women who received aspirin or no intervention.
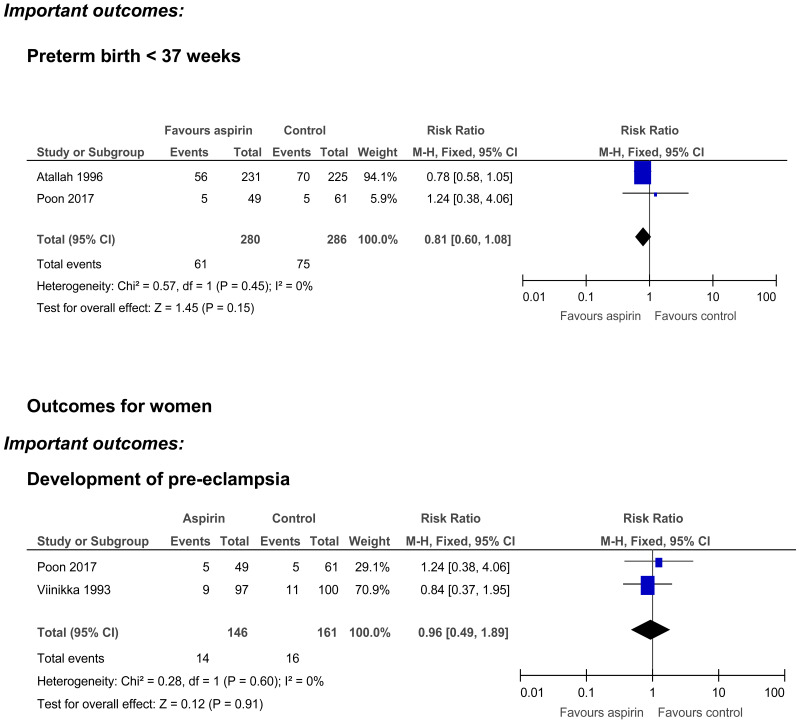
Preterm birth <37 weeks
- Two randomised controlled trials (n=566) provided low quality evidence to show that there was no clinically important difference in in the number of preterm births (<37 weeks) for women who received aspirin, as compared to those who received no intervention
- A meta-analysis of individual participant data from a further 17 RCTs (n=2518) provided moderate quality evidence to show there may be a clinically important reduction in the number of preterm births (<37 weeks) for women who received aspirin, but there was some uncertainty over the estimate (RR 0.73, 95% CI 0.53 to 1.00).
Preterm birth <34 weeks
- A meta-analysis of individual participant data from 17 RCTs (n=2518) provided low quality evidence to show no clinically important difference in the number of preterm births (<34 weeks) between those who received aspirin or no intervention.
Preterm birth <28 weeks
- A meta-analysis of individual participant data from 17 RCTs (n=2518) provided low quality evidence to show no clinically important difference in the number of preterm births (<28 weeks) between those who received aspirin or no intervention.
Admission to neonatal unit
- One randomised controlled trial (n=197) provided low quality evidence to show a clinically important reduction in the number of neonatal unit admissions for babies born to women who received aspirin, as compared to those who received no intervention.
Outcomes for women
Critical outcomes
Severe hypertension
- One randomised controlled trial (n=197) provided very low quality evidence to show no clinically important difference in the occurrence of worsening hypertension between those who received aspirin or no intervention.
- One randomised controlled trial (n=197) provided moderate quality evidence to show no clinically important difference in the diastolic blood pressure at 36 weeks’ gestation between those who received aspirin or no intervention.
Important outcomes
Development of pre-eclampsia
- Two randomised controlled trials (n=307) provided very low quality evidence to show no clinically important difference in the development of pre-eclampsia between those who received aspirin or no intervention.
- A meta-analysis of individual participant data from 31 RCTs (n=3303) provided high quality evidence to show no clinically important difference in the development of pre-eclampsia between those who received aspirin or no intervention.
Spontaneous onset of labour
- One randomised controlled trial (n=197) provided low quality evidence to show no clinically important difference in the number of women who had a spontaneous onset of labour between those who received aspirin or no intervention.
See appendix E for Forest plots.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
Treatment of chronic hypertension in pregnancy aims to control the mother’s blood pressure without leading to any adverse effects on the baby. The committee therefore identified 3 outcomes of critical importance to allow the balance of benefits and harms of interventions to be assessed. These were control of blood pressure (outcome for women) and perinatal mortality (including stillbirth and neonatal death) and small for gestational age (both outcomes for babies).
The committee also identified 7 important outcomes for babies to provide further information on the potential harms to babies. These were birth weight, gestational age at birth, preterm birth (< 28 weeks, <34 weeks, <37 weeks), admission to a neonatal unit, cerebral palsy, neurodevelopmental delay, and neurosensory impairment. Six further important outcomes for women with chronic hypertension were identified, and these were superimposed pre-eclampsia, HELLP, placental abruption, onset of labour, mode of birth, and maternal death.
The quality of the evidence
Eighteen articles were included in the review. The quality of the evidence was assessed with the Cochrane Risk of Bias tool and ranged from moderate to very low. The main sources of potential bias were: lack of information on the randomisation method used, unreported or unclear concealment of allocation, and lack of blinding of participants and investigators.
The committee determined that there was sufficient evidence to allow them to make some recommendations relating to treatment initiation thresholds and treatment targets. However, there was not enough evidence to discriminate between different pharmacologic treatments, therefore they made a research recommendation relating to the choice of pharmacologic agents. There was also concern (based on the committee’s clinical knowledge and expertise) over the potential neonatal adverse outcomes with the use of beta-blockers in women with hypertension, and so the committee made a research recommendation relating to this too.
Benefits and harms
The committee made an overarching recommendation on the advice that should be provided to pregnant women with chronic hypertension, in accordance with existing NICE guidelines on the treatment of hypertension in adults. This guideline does not provide specific advice for pregnant women, but the committee agreed that the principles of treatment and advice (such as exercise and healthy diet) are similar.
No specific evidence was available that demonstrated the blood pressure at which treatment for chronic hypertension should be initiated, but the committee identified that in the CHIPS study (Magee 2015) (which had identified that tight blood pressure control led to a reduced incidence of severe hypertension in women), the treatment threshold had been a diastolic blood pressure of ≥90mmHg. There was no equivalent systolic blood pressure treatment threshold in this study so the committee referred to the NICE guidelines on the treatment of hypertension in adults and used their treatment threshold of ≥140mmHg. Similarly, for the target blood pressure the committee adopted the CHIPS target of ≤85mmHg diastolic and the adult guideline target of ≤135mmHg systolic. There was some low quality evidence that a tighter control of blood pressure may slightly increase the number of babies who were small-for-gestational-age (but with no impact on need for high-level neonatal care or pregnancy loss). However, the committee noted that in the full CHIPS trial (including women with both chronic hypertension and gestational hypertension) no difference was seen in the number of babies who were born small for gestational age, after adjustment for baseline differences between the two groups of participants. Overall, the committee balanced the benefits and harms and made recommendations to adopt these treatment thresholds and treatment targets.
Chronic hypertension is associated with complications during pregnancy, including adverse maternal and neonatal outcomes. However, treatments such as antihypertensives and aspirin also carry potential risks such as side effects for the mother and the possibility of teratogenic effects. Clinicians continuing existing treatment or initiating treatments should inform women of these risks and benefits. There was evidence for beneficial effects on the mother’s blood pressure with tight blood pressure control. There was no evidence of a benefit on placental abruption or preterm birth with any of the interventions, but some evidence for a reduction in stillbirths and increased gestational age at birth with some of the pharmacologic interventions. However, there was also some evidence for harm with interventions – a possible increase in small-for-gestational age babies with tight blood pressure control and atenolol. The committee weighed up the benefits and harms and, based on their clinical expertise as well, agreed that treatment with antihypertensive medication should be continued or initiated in pregnant women with chronic hypertension, in order to reduce the risk of serious complications such as severe hypertension, placental abruption or preterm birth.
The available evidence was not sufficient to recommend one antihypertensive medicine over another as it demonstrated no significant differences between labetalol, nifedipine and methyldopa. The only significant difference noted was a small increase in gestational age at delivery for infants of mothers treated with labetalol, as compared with nifedipine. However, the committee noted that this difference was not seen after adjustment for baseline differences in the treatment groups. When methyldopa was compared with no intervention or placebo, it showed that those who received the active intervention experienced a longer gestational age and fewer stillbirths. The committee discussed the fact that labetalol was specifically licensed in pregnancy (after the 1st trimester) whereas other treatments are not, but that all three medicines had been used in pregnant women for many years with no reports of major adverse effects, had been recommended in the 2010 guideline for gestational hypertension and pre-eclampsia, and that it made sense for clinicians to use the same range of drugs to treat all types of hypertension. The committee therefore chose to recommend labetaolol as the first-line choice due to its licensed status, with nifedipine or methylodopa as alternative treatment options
Aspirin had been included as one of the interventions in the review and there was evidence to show that it may reduce preterm birth (<37 weeks) and neonatal unit admission. The committee therefore chose to retain the recommendation from the previous guideline to use aspirin from the second trimester of pregnancy (12 weeks). The committee noted that the studies used different doses of aspirin, ranging from 50 to 150mg daily, and that common practice in the UK was to offer 75 to 150mg, with there being little evidence to support the optimal dose.
Because of the lack of evidence on the effectiveness and safety of antihypertensives in pregnant women with chronic hypertension, the committee decided to repeat the research recommendation made in the previous version of the guideline, to determine the best agent to use. The committee agreed that as ethnicity has an impact on the choice of antihypertensives outside of pregnancy, this study should include an analysis by different ethnicities.
Labetalol is approved for use in pregnancy, and atenolol had shown some efficacy for blood pressure control but with very limited evidence and possibly some adverse effects. The committee were aware from their own clinical experience and knowledge that these adverse effects included hypoglycaemia, but as there is limited data for both of these medicines, the committee also made a research recommendation to establish whether beta-blockers (and mixed alpha-beta blockers) can be used safely in chronic hypertension in pregnancy.
The committee noted that since the previous guideline had been published, NICE had produced diagnostic guidance on the use of placental growth factor (PlGF) monitoring to help rule-out pre-eclampsia in women between 20+0 and 34+6 weeks. Since chronic hypertension is a risk factor for pre-eclampsia, the committee agreed that a cross-reference to this guidance should be included.
Cost effectiveness and resource use
No relevant studies were identified in a systematic review of the economic evidence.
The committee considered that these recommendations would not lead to an increase in resource use as they reflect standard practice for the majority of centres.
Other factors the committee took into account
The committee were aware of the findings from a recently updated Cochrane systematic review and meta-analysis on antihypertensive treatment in pregnancy, which indicated that beta-blockers and calcium channel blockers were more effective than methyldopa at preventing severe hypertension. The Cochrane review included a mixed population of women with any hypertension during pregnancy and so did not meet the protocol criteria for inclusion in this evidence report (which included women with chronic hypertension only). However, the committee agreed that it would be appropriate to recommend methyldopa as the third-line option, after labetalol and nifedipine, based on the findings of the Cochrane review and their experience of the side-effect profile of methyldopa.
The committee were also aware of 2 forthcoming studies which may provide further evidence in this area. The Chronic Hypertension and Pregnancy (CHAP) study will provide further advice on treatment initiation thresholds (estimated completion date December 2019) and the When to Induce Labour to Limit risk in pregnancy hypertension (WILL) study is investigating the optimal timing of birth.
The committee were aware of a recent publication from NHS England, Saving Babies’ Lives, which recommended the use of low dose aspirin in higher risk women. The dose suggested in this document was 150mg at night, or lower doses (60 to 75 mg) in some circumstances, for example women with hepatic or renal disease. This corresponded with the range of 75mg to 150 mg suggested by the committee.
References
Atallah 1996
Atallah AN, Collins R, Farrell B, Handoll H, Freitas A, Kinsui L, Fukushima O, Amorim M, Eduardo R, Durante A, Vieira C. ECPPA: randomised trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnant women. British Journal of Obstetrics and Gynaecology 1996;103(1):39–47. [PubMed: 8608096]Askie 2007
Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791–8. [PubMed: 17512048]Butters 1990
Butters L, Kennedy S, Rubin PC. Atenolol in essential hypertension during pregnancy. British Medical Journal 1990;301(6752):587–9. [PMC free article: PMC1663720] [PubMed: 2242456]Cockburn 1982
Cockburn J, Ounsted M, Moar VA, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet 1982;319(8273):647–9. [PubMed: 6121965]Hamed 2014
Hamed HO, Alsheeha MA, Abu‐Elhasan AM, Abd Elmoniem AE, Kamal MM. Pregnancy outcomes of expectant management of stable mild to moderate chronic hypertension as compared with planned delivery. International Journal of Gynecology & Obstetrics 2014;127(1):15–20. [PubMed: 24957533]Kasawara 2013
Kasawara KT, Burgos CS, do Nascimento SL, Ferreira NO, Surita FG, Pinto e Silva JL. Maternal and perinatal outcomes of exercise in pregnant women with chronic hypertension and/or previous preeclampsia: a randomized controlled trial. ISRN Obstetrics and Gynecology 2013;doi:10.1155/2013/857047 [PMC free article: PMC3753734] [PubMed: 23997960] [CrossRef]Magee 2015
Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Singer J, Gafni A, Gruslin A. Less-tight versus tight control of hypertension in pregnancy. New England Journal of Medicine 2015;372(5):407–17. [PubMed: 25629739]Moore 1982
Moore MP, Redman CW. The treatment of hypertension in pregnancy. Current Medical Research and Opinion 1982;8(sup1):39–46.Moore 2015
Moore GS, Allshouse AA, Post AL, Galan HL, Heyborne KD. Early initiation of low-dose aspirin for reduction in preeclampsia risk in high-risk women: a secondary analysis of the MFMU High-Risk Aspirin Study. Journal of Perinatology. 2015 May;35(5):328. [PMC free article: PMC4838902] [PubMed: 25474553]Parazzini 1993
Parazzini F, Benedetto C, Frusca T, Gregorini G, Bocciolone L, Marozio L, Romero M, Danesino V, De Gaetano G, Gastaldi A, Massobrio M. Low‐dose aspirin in prevention and treatment of intrauterine growth retardation and pregnancy‐induced hypertension. International Journal of Gynecology & Obstetrics. 1993;43(1):91-.Poon 2017
Poon LC, Wright D, Rolnik DL, Syngelaki A, Delgado JL, Tsokaki T, Leipold G, Akolekar R, Shearing S, De Stefani L, Jani JC. Aspirin for Evidence-Based Preeclampsia Prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. American Journal of Obstetrics & Gynecology 2017;217(5):585–e1. [PubMed: 28784417]Redman 1976
Redman CW, Beilin LJ, Bonnar J, Ounsted MK. Fetal outcome in trial of antihypertensive treatment in pregnancy. Lancet 1976;308(7989):753–6. [PubMed: 61439]Sibai 1990
Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. American Journal of Obstetrics & Gynecology 1990;162(4):960–7. [PubMed: 2183619]van Vliet 2017
van Vliet EO, Askie LA, Mol BW, Oudijk MA. Antiplatelet agents and the prevention of spontaneous preterm birth: a systematic review and meta-analysis. Obstetrics & Gynecology. 2017;129(2):327–36. [PubMed: 28079785]Vigil-De Gracia 2014
Vigil-De Gracia P, Dominguez L, Solis A. Management of chronic hypertension during pregnancy with furosemide, amlodipine or aspirin: a pilot clinical trial. The Journal of Maternal-Fetal & Neonatal Medicine. 2014;27(13):1291–4. [PubMed: 24102416]Viinikka 1993
Viinikka L, Hartikainen‐Sorri AL, Lumme R, Hiilesmaa V, Ylikorkala O. Low dose aspirin in hypertensive pregnant women: effect on pregnancy outcome and prostacyclin‐thromboxane balance in mother and newborn. British Journal of Obstetrics & Gynaecology 1993 100(9):809–15. [PubMed: 8217999]Webster 2017
Webster LM, Myers JE, Nelson-Piercy C, Harding K, Cruickshank JK, Watt-Coote I, Khalil A, Wiesender C, Seed PT, Chappell LC. Labetalol Versus Nifedipine as Antihypertensive Treatment for Chronic Hypertension in Pregnancy: A Randomized Controlled Trial. Hypertension 2017:HYPERTENSIONAHA-117. [PubMed: 28893900]Weitz 1987
Weitz C, Khouzami V, Maxwell K, Johnson JW. Treatment of hypertension in pregnancy with methyldopa: a randomized double blind study. International Journal of Gynecology & Obstetrics 1987;25(1):35–40. [PubMed: 2883043]
Appendices
Appendix A. Review protocol
Table 3Review protocol
| Field (based on PRISMA-P) | Content |
|---|---|
| Key area in the scope | Management of pregnancy with chronic hypertension |
| Draft review question from the previous guideline | What interventions for chronic hypertension are effective at improving outcomes for women and infants? |
| Actual review question | What interventions for chronic hypertension are effective at improving outcomes for women and infants? |
| Type of review question | Intervention |
| Objective of the review | To update the recommendations in the previous guideline (CG107) for the treatment of pregnant women who have chronic hypertension – surveillance has indicated that the CHIPS study may have changed treatment targets (rec 1.2.3.1) |
| Eligibility criteria – population/disease/condition/issue/domain | Pregnant women with chronic hypertension. This population includes women with:
|
| Eligibility criteria – intervention(s)/exposure(s)/prognostic factor(s) |
|
| Eligibility criteria – comparator(s)/control or reference (gold) standard |
|
| Outcomes and prioritisation |
Outcomes for babies: Critical outcomes:
Critical outcome:
|
| Eligibility criteria – study design | Only published full text papers in English language
|
| Exclusion criteria |
|
| Proposed stratified, sensitivity/sub-group analysis, or meta-regression |
Stratify for mild/mod/severe hypertension Stratify for black ethnic group Stratify by the following types of interventions:
Sub-groups in case of heterogeneity for individual drugs |
| Selection process – duplicate screening/selection/analysis | Duplicate screening/selection/analysis will not be undertaken for this review as this question was not prioritised for it. Included and excluded studies will be cross checked with the committee and with published systematic reviews when available. |
| Data management (software) |
If pairwise meta-analyses are undertaken, they will be performed using Cochrane Review Manager (RevMan5). ‘GRADE’ will be used to assess the quality of evidence for each outcome. STAR will be used for bibliographies/citations, text mining, and study sifting, data extraction and quality assessment/critical appraisal. |
| Information sources – databases and dates |
Sources to be searched: Medline, Medline In-Process, CCTR, CDSR, DARE, HTA and Embase. Limits (e.g. date, study design): Study design limited to Systematic Reviews, RCTs and Comparative Cohort Studies. Apply standard animal/non-English language filters. No date limit. Supplementary search techniques: No supplementary search techniques were used. Key papers (from surveillance report):
|
| Identify if an update | This is an update. Studies meeting the current protocol criteria and previously included in the 2010 guideline (CG107) will be included in this update. |
| Author contacts |
Developer: National Guideline Alliance |
| Highlight if amendment to previous protocol |
New items added in this protocol: New interventions were integrated to reflect those highlighted by the surveillance summary. These were: timing of delivery, tight versus less tight control, monitoring of blood pressure and exercise. New outcomes: neonatal death up to 7 days, neurodevelopmental outcomes Items removed from the previous protocol: Removed from the interventions: thiazide, dypiridamole, rest and bed rest were deleted. The population, comparisons and outcomes are the same as in the 2010 protocol for this review question. |
| Search strategy – for one database | For details please see appendix B |
| Data collection process – forms/duplicate | Studies included in the previous guideline (CG107) that meet the inclusion criteria of this protocol will be re-extracted in a standardised evidence table and published as appendix D (clinical evidence tables) or H (economic evidence tables). |
| Data items – define all variables to be collected | For clinical evidence tables (appendix D), the following data items will be collected: full reference, study ID, type of study, objective country/ies where the study was carried out, inclusion criteria, exclusion criteria, methods, results and limitations. |
| Methods for assessing bias at outcome/study level |
Appraisal of methodological quality: The methodological quality of each study will be assessed using an appropriate checklist:
The risk of bias across all available evidence will evaluated for each outcome using an adaptation of the ‘Grading of Recommendations Assessment, Development and Evaluation (GRADE) toolbox’ developed by the international GRADE working group http://www Studies included in the previous guideline (CG107) that meet the inclusion criteria of this protocol will be assessed with the above mentioned checklists (as appropriate) and outcomes will be evaluated using GRADE. |
| Criteria for quantitative synthesis | For details please see section 6.4 of Developing NICE guidelines: the manual |
| Methods for quantitative analysis – combining studies and exploring (in)consistency |
Synthesis of data: Meta-analysis will be conducted where appropriate using Review Manager. Minimum important differences: Default values will be used of: 0.8 and 1.25 for dichotomous outcomes; 0.5 times SD for continuous outcomes, unless more appropriate values are identified by the guideline committee or in the literature. Double sifting, data extraction and methodological quality assessment: Sifting, data extraction, appraisal of methodological quality and GRADE assessment will be performed by the systematic reviewer. Quality control will be performed by the senior systematic reviewer. Dual quality assessment and data extraction will not be performed. How the evidence included in the previous guideline will be incorporated with the new evidence: Studies meeting the current protocol criteria and previously included in the 2010 guideline (CG107) will be included in this update. The methods for quantitative analysis – combining studies and exploring (in) consistency- will be the same as for the new evidence (see above). |
| Meta-bias assessment – publication bias, selective reporting bias | For details please see section 6.2 of Developing NICE guidelines: the manual. |
| Confidence in cumulative evidence | For details please see sections 6.4 and 9.1 of Developing NICE guidelines: the manual |
| Rationale/context – what is known | For details please see the introduction to the evidence review. |
| Describe contributions of authors and guarantor |
A multidisciplinary committee developed the guideline. The committee was convened by the National Guideline Alliance and chaired by Sarah Fishburn in line with section 3 of Developing NICE guidelines: the manual. Staff from the National Guideline Alliance undertook systematic literature searches, appraised the evidence, conducted meta-analysis and cost-effectiveness analysis where appropriate, and drafted the guideline in collaboration with the committee. For details please see the methods chapter of the full guideline. |
| Sources of funding/support | The National Guideline Alliance is funded by NICE and hosted by the Royal College of Obstetricians and Gynaecologists |
| Name of sponsor | The National Guideline Alliance is funded by NICE and hosted by the Royal College of Obstetricians and Gynaecologists |
| Roles of sponsor | NICE funds the National Guideline Alliance to develop guidelines for the NHS in England. |
| PROSPERO registration number | Not registered with PROSPERO |
Appendix B. Literature search strategies
Databases: Medline; Medline EPub Ahead of Print; and Medline In-Process & Other Non-Indexed Citations
Date of last search: 19/02/18
| # | Searches |
|---|---|
| 1 | META-ANALYSIS/ |
| 2 | META-ANALYSIS AS TOPIC/ |
| 3 | (meta analy* or metanaly* or metaanaly*).ti,ab. |
| 4 | ((systematic* or evidence*) adj2 (review* or overview*)).ti,ab. |
| 5 | (reference list* or bibliograph* or hand search* or manual search* or relevant journals).ab. |
| 6 | (search strategy or search criteria or systematic search or study selection or data extraction).ab. |
| 7 | (search* adj4 literature).ab. |
| 8 | (medline or pubmed or cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or science citation index or bids or cancerlit).ab. |
| 9 | cochrane.jw. |
| 10 | or/1-9 |
| 11 | randomized controlled trial.pt. |
| 12 | controlled clinical trial.pt. |
| 13 | pragmatic clinical trial.pt. |
| 14 | randomi#ed.ab. |
| 15 | placebo.ab. |
| 16 | randomly.ab. |
| 17 | CLINICAL TRIALS AS TOPIC/ |
| 18 | trial.ti. |
| 19 | or/11-18 |
| 20 | COHORT STUDIES/ |
| 21 | (cohort adj3 (study or studies)).ti,ab. |
| 22 | (Cohort adj3 analy$).ti,ab. |
| 23 | FOLLOW-UP STUDIES/ |
| 24 | (Follow$ up adj3 (study or studies)).ti,ab. |
| 25 | LONGITUDINAL STUDIES/ |
| 26 | longitudinal$.ti,ab. |
| 27 | PROSPECTIVE STUDIES/ |
| 28 | prospective$.ti,ab. |
| 29 | RETROSPECTIVE STUDIES/ |
| 30 | retrospective$.ti,ab. |
| 31 | OBSERVATIONAL STUDY/ |
| 32 | observational$.ti,ab. |
| 33 | or/20-32 |
| 34 | HYPERTENSION, PREGNANCY-INDUCED/ |
| 35 | PREGNANCY/ and HYPERTENSION/ |
| 36 | PRE-ECLAMPSIA/ |
| 37 | HELLP SYNDROME/ |
| 38 | ((pregnan$ or gestation$) adj5 hypertensi$).ti. |
| 39 | preeclamp$.ti,ab. |
| 40 | pre eclamp$.ti,ab. |
| 41 | HELLP.ti,ab. |
| 42 | tox?emi$.ti,ab. |
| 43 | or/34-42 |
| 44 | exp ADRENERGIC ALPHA-2 RECEPTOR AGONISTS/ |
| 45 | (alpha$ adj3 Agonist?).mp. |
| 46 | (Brimonidine Tartrate or Clonidine or exmedetomidine or Guanabenz or Guanfacine or Medetomidine or Methyldopa or Xylazine).mp. |
| 47 | exp ADRENERGIC BETA-ANTAGONISTS/ |
| 48 | (Adrenergic beta$ adj3 Antagonist?).mp. |
| 49 | Beta blocker?.mp. |
| 50 | (mixed adj3 blocker?).ti,ab. |
| 51 | (Alprenolol or Bunolol or Bupranolol or Carteolol or Dihydroalprenolol or Iodocyanopindolol or Labetalol or Levobunolol or Metipranolol or Nadolol or Oxprenolol or Penbutolol or Pindolol or Propranolol or Sotalol or Timolol or Acebutolol or Atenolol or Betaxolol or Bisoprolol or Celiprolol or Metoprolol or Practolol or Butoxamine).mp. |
| 52 | exp CALCIUM CHANNEL BLOCKERS/ |
| 53 | (calcium channel adj3 (blocker? or antagonist?)).ti,ab. |
| 54 | (Amlodipine or Amrinone or Bencyclane or Bepridil or Cinnarizine or Conotoxin? or Diltiazem or Felodipine or Fendiline or Flunarizine or Gallopamil or Isradipine or Lidoflazine or Mibefradil or Nicardipine or Nifedipine or Nimodipine or Nisoldipine or Nitrendipine or Perhexiline or Pregabalin or Prenylamine or Risedronate Sodium or Tiapamil Hydrochloride or Verapamil or omega-Agatoxin IVA or omega-Conotoxin?).mp. |
| 55 | Magnesium Sulfate.ti. |
| 56 | Magnesium Sulfate.ab. /freq=2 |
| 57 | DIURETICS/ |
| 58 | diuretic?.ti,ab. |
| 59 | (Acetazolamide or Amiloride or Bendroflumethiazide or Bumetanide or Chlorothiazide or Chlorthalidone or Clopamide or Cyclopenthiazide or Ethacrynic Acid or Ethoxzolamide or Furosemide or Hydrochlorothiazide or Hydroflumethiazide or Indapamide or Mefruside or Methazolamide or Methyclothiazide or Metolazone or Muzolimine or Polythiazide or Potassium Citrate or Spironolactone or Ticrynafen or Triamterene or Trichlormethiazide or Xipamide or Isosorbide or Mannitol or Canrenoic Acid or Canrenone).mp. |
| 60 | exp ANGIOTENSIN-CONVERTING ENZYME INHIBITORS/ |
| 61 | (angiotensin converting enzyme adj3 (antagonist? or inhibitor?)).ti,ab. |
| 62 | (ACE adj3 (antagonist? or inhibitor?)).ti,ab. |
| 63 | (Captopril or Cilazapril or Enalapril or Enalaprilat or Fosinopril or Lisinopril or Perindopril or Ramipril or Teprotide).mp. |
| 64 | ASPIRIN/ |
| 65 | (acetylsalicylic acid or aspirin?).ti. |
| 66 | (acetylsalicylic acid or aspirin?).ab. /freq=2 |
| 67 | ((elect$ or plan$) adj3 deliver$).ti,ab. |
| 68 | (expect$ adj3 manag$).ti,ab. |
| 69 | (tight$ adj3 (manag$ or control$)).ti,ab. |
| 70 | BLOOD PRESSURE DETERMINATION/ |
| 71 | BLOOD PRESSURE MONITORING, AMBULATORY/ |
| 72 | ((Automat$ or ambulatory or self$) adj3 blood pressure?).ti,ab. |
| 73 | exp EXERCISE/ |
| 74 | (exercis$ or physical$ activ$ or swim$ or cycl$ or sport? or run$ or jog$ or walk$ or stair climb$ or gym$ or resistance train$ or yoga or pilates).ti. |
| 75 | (exercis$ or physical$ activ$ or swim$ or cycl$ or sport? or run$ or jog$ or walk$ or stair climb$ or gym$ or resistance train$ or yoga or pilates).ab. /freq=2 |
| 76 | exp DIET/ |
| 77 | diet$.ti. |
| 78 | diet$.ab. /freq=2 |
| 79 | (calor$ adj3 restrict$).ti,ab. |
| 80 | ((portion? or serving) adj3 size?).ti,ab. |
| 81 | ((low$ or restrict$) adj3 (salt or sodium)).ti,ab. |
| 82 | or/44-81 |
| 83 | 43 and 82 |
| 84 | limit 83 to english language |
| 85 | LETTER/ |
| 86 | EDITORIAL/ |
| 87 | NEWS/ |
| 88 | exp HISTORICAL ARTICLE/ |
| 89 | ANECDOTES AS TOPIC/ |
| 90 | COMMENT/ |
| 91 | CASE REPORT/ |
| 92 | (letter or comment*).ti. |
| 93 | or/85-92 |
| 94 | RANDOMIZED CONTROLLED TRIAL/ or random*.ti,ab. |
| 95 | 93 not 94 |
| 96 | ANIMALS/ not HUMANS/ |
| 97 | exp ANIMALS, LABORATORY/ |
| 98 | exp ANIMAL EXPERIMENTATION/ |
| 99 | exp MODELS, ANIMAL/ |
| 100 | exp RODENTIA/ |
| 101 | (rat or rats or mouse or mice).ti. |
| 102 | or/95-101 |
| 103 | 84 not 102 |
| 104 | 10 and 103 |
| 105 | 19 and 103 |
| 106 | 33 and 103 |
| 107 | or/104-106 |
Database: Embase; and Embase Classic
Date of last search: 19/02/18
| # | Searches |
|---|---|
| 1 | SYSTEMATIC REVIEW/ |
| 2 | META-ANALYSIS/ |
| 3 | (meta analy* or metanaly* or metaanaly*).ti,ab. |
| 4 | ((systematic or evidence) adj2 (review* or overview*)).ti,ab. |
| 5 | (reference list* or bibliograph* or hand search* or manual search* or relevant journals).ab. |
| 6 | (search strategy or search criteria or systematic search or study selection or data extraction).ab. |
| 7 | (search* adj4 literature).ab. |
| 8 | (medline or pubmed or cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or science citation index or bids or cancerlit).ab. |
| 9 | ((pool* or combined) adj2 (data or trials or studies or results)).ab. |
| 10 | cochrane.jw. |
| 11 | or/1-10 |
| 12 | random*.ti,ab. |
| 13 | factorial*.ti,ab. |
| 14 | (crossover* or cross over*).ti,ab. |
| 15 | ((doubl* or singl*) adj blind*).ti,ab. |
| 16 | (assign* or allocat* or volunteer* or placebo*).ti,ab. |
| 17 | CROSSOVER PROCEDURE/ |
| 18 | SINGLE BLIND PROCEDURE/ |
| 19 | RANDOMIZED CONTROLLED TRIAL/ |
| 20 | DOUBLE BLIND PROCEDURE/ |
| 21 | or/12-20 |
| 22 | COHORT ANALYSIS/ |
| 23 | (cohort adj3 (study or studies)).ti,ab. |
| 24 | (Cohort adj3 analy$).ti,ab. |
| 25 | FOLLOW UP/ |
| 26 | (Follow$ up adj3 (study or studies)).ti,ab. |
| 27 | LONGITUDINAL STUDY/ |
| 28 | longitudinal$.ti,ab. |
| 29 | PROSPECTIVE STUDY/ |
| 30 | prospective$.ti,ab. |
| 31 | RETROSPECTIVE STUDY/ |
| 32 | retrospective$.ti,ab. |
| 33 | OBSERVATIONAL STUDY/ |
| 34 | observational$.ti,ab. |
| 35 | or/22-34 |
| 36 | MATERNAL HYPERTENSION/ |
| 37 | PREGNANCY/ and HYPERTENSION/ |
| 38 | PREECLAMPSIA/ |
| 39 | HELLP SYNDROME/ |
| 40 | ((pregnan$ or gestation$) adj5 hypertensi$).ti. |
| 41 | preeclamp$.ti,ab. |
| 42 | pre eclamp$.ti,ab. |
| 43 | HELLP.ti,ab. |
| 44 | tox?emi$.ti,ab. |
| 45 | or/36-44 |
| 46 | exp *ALPHA 2 ADRENERGIC RECEPTOR STIMULATING AGENT/ |
| 47 | (alpha$ adj3 Agonist?).mp. |
| 48 | (Brimonidine Tartrate or Clonidine or exmedetomidine or Guanabenz or Guanfacine or Medetomidine or Methyldopa or Xylazine).mp. |
| 49 | exp *BETA ADRENERGIC RECEPTOR BLOCKING AGENT/ |
| 50 | (adrenergic adj3 beta adj3 antagonist?).ti,ab. |
| 51 | Beta blocker?.mp. |
| 52 | (mixed adj3 blocker?).ti,ab. |
| 53 | (Alprenolol or Bunolol or Bupranolol or Carteolol or Dihydroalprenolol or Iodocyanopindolol or Labetalol or Levobunolol or Metipranolol or Nadolol or Oxprenolol or Penbutolol or Pindolol or Propranolol or Sotalol or Timolol or Acebutolol or Atenolol or Betaxolol or Bisoprolol or Celiprolol or Metoprolol or Practolol or Butoxamine).mp. |
| 54 | exp *CALCIUM CHANNEL BLOCKING AGENT/ |
| 55 | (calcium channel adj3 (blocker? or antagonist?)).ti,ab. |
| 56 | (Amlodipine or Amrinone or Bencyclane or Bepridil or Cinnarizine or Conotoxin? or Diltiazem or Felodipine or Fendiline or Flunarizine or Gallopamil or Isradipine or Lidoflazine or Mibefradil or Nicardipine or Nifedipine or Nimodipine or Nisoldipine or Nitrendipine or Perhexiline or Pregabalin or Prenylamine or Risedronate Sodium or Tiapamil Hydrochloride or Verapamil or omega-Agatoxin IVA or omega-Conotoxin?).mp. |
| 57 | Magnesium Sulfate.ti. |
| 58 | Magnesium Sulfate.ab. /freq=2 |
| 59 | exp *DIURETIC AGENT/ |
| 60 | diuretic?.ti,ab. |
| 61 | (Acetazolamide or Amiloride or Bendroflumethiazide or Bumetanide or Chlorothiazide or Chlorthalidone or Clopamide or Cyclopenthiazide or Ethacrynic Acid or Ethoxzolamide or Furosemide or Hydrochlorothiazide or Hydroflumethiazide or Indapamide or Mefruside or Methazolamide or Methyclothiazide or Metolazone or Muzolimine or Polythiazide or Potassium Citrate or Spironolactone or Ticrynafen or Triamterene or Trichlormethiazide or Xipamide or Isosorbide or Mannitol or Canrenoic Acid or Canrenone).mp. |
| 62 | exp *DIPEPTIDYL CARBOXYPEPTIDASE INHIBITOR/ |
| 63 | (angiotensin converting enzyme adj3 (antagonist? or inhibitor?)).ti,ab. |
| 64 | (ACE adj3 (antagonist? or inhibitor?)).ti,ab. |
| 65 | (Captopril or Cilazapril or Enalapril or Enalaprilat or Fosinopril or Lisinopril or Perindopril or Ramipril or Teprotide).mp. |
| 66 | *ACETYLSALICYLIC ACID/ |
| 67 | (acetylsalicylic acid or aspirin?).ti. |
| 68 | (acetylsalicylic acid or aspirin?).ab. /freq=2 |
| 69 | ((elect$ or plan$) adj3 deliver$).ti,ab. |
| 70 | (expect$ adj3 manag$).ti,ab. |
| 71 | (tight$ adj3 (manag$ or control$)).ti,ab. |
| 72 | *BLOOD PRESSURE MEASUREMENT/ |
| 73 | *BLOOD PRESSURE MONITORING/ |
| 74 | ((Automat$ or ambulatory or self$) adj3 blood pressure?).ti,ab. |
| 75 | exp *EXERCISE/ |
| 76 | (exercis$ or physical$ activ$ or swim$ or cycl$ or sport? or run$ or jog$ or walk$ or stair climb$ or gym$ or resistance train$ or yoga or pilates).ti. |
| 77 | (exercis$ or physical$ activ$ or swim$ or cycl$ or sport? or run$ or jog$ or walk$ or stair climb$ or gym$ or resistance train$ or yoga or pilates).ab. /freq=2 |
| 78 | exp *DIET/ |
| 79 | diet$.ti. |
| 80 | diet$.ab. /freq=2 |
| 81 | (calor$ adj3 restrict$).ti,ab. |
| 82 | ((portion? or serving) adj3 size?).ti,ab. |
| 83 | *SODIUM RESTRICTION/ |
| 84 | ((low$ or restrict$) adj3 (salt or sodium)).ti,ab. |
| 85 | or/46-84 |
| 86 | 45 and 85 |
| 87 | limit 86 to english language |
| 88 | letter.pt. or LETTER/ |
| 89 | note.pt. |
| 90 | editorial.pt. |
| 91 | CASE REPORT/ or CASE STUDY/ |
| 92 | (letter or comment*).ti. |
| 93 | or/88-92 |
| 94 | RANDOMIZED CONTROLLED TRIAL/ or random*.ti,ab. |
| 95 | 93 not 94 |
| 96 | ANIMAL/ not HUMAN/ |
| 97 | NONHUMAN/ |
| 98 | exp ANIMAL EXPERIMENT/ |
| 99 | exp EXPERIMENTAL ANIMAL/ |
| 100 | ANIMAL MODEL/ |
| 101 | exp RODENT/ |
| 102 | (rat or rats or mouse or mice).ti. |
| 103 | or/95-102 |
| 104 | 87 not 103 |
| 105 | 11 and 104 |
| 106 | 21 and 104 |
| 107 | 35 and 104 |
| 108 | or/105-107 |
Databases: Cochrane Central Register of Controlled Trials; Cochrane Database of Systematic Reviews; Database of Abstracts of Reviews of Effects; and Health Technology Assessment
Date of last search: 19/02/18
| # | Searches |
|---|---|
| 1 | MeSH descriptor: [HYPERTENSION, PREGNANCY-INDUCED] this term only |
| 2 | MeSH descriptor: [PREGNANCY] this term only |
| 3 | MeSH descriptor: [HYPERTENSION] this term only |
| 4 | #2 and #3 |
| 5 | MeSH descriptor: [PRE-ECLAMPSIA] this term only |
| 6 | MeSH descriptor: [HELLP SYNDROME] this term only |
| 7 | ((pregnan* or gestation*) near/5 hypertensi*):ti |
| 8 | preeclamp*:ti,ab |
| 9 | pre eclamp*:ti,ab |
| 10 | HELLP:ti,ab |
| 11 | tox?emi*:ti,ab |
| 12 | #1 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 |
| 13 | MeSH descriptor: [ADRENERGIC ALPHA-2 RECEPTOR AGONISTS] 2 tree(s) exploded |
| 14 | (alpha* near/3 Agonist?):ti,ab |
| 15 | (Brimonidine Tartrate or Clonidine or exmedetomidine or Guanabenz or Guanfacine or Medetomidine or Methyldopa or Xylazine):ti,ab |
| 16 | MeSH descriptor: [ADRENERGIC BETA-ANTAGONISTS] 2 tree(s) exploded |
| 17 | (Adrenergic beta* near/3 Antagonist?):ti,ab |
| 18 | Beta blocker?:ti,ab |
| 19 | (mixed near/3 blocker?):ti,ab |
| 20 | (Alprenolol or Bunolol or Bupranolol or Carteolol or Dihydroalprenolol or Iodocyanopindolol or Labetalol or Levobunolol or Metipranolol or Nadolol or Oxprenolol or Penbutolol or Pindolol or Propranolol or Sotalol or Timolol or Acebutolol or Atenolol or Betaxolol or Bisoprolol or Celiprolol or Metoprolol or Practolol or Butoxamine):ti,ab |
| 21 | MeSH descriptor: [CALCIUM CHANNEL BLOCKERS] 2 tree(s) exploded |
| 22 | (calcium channel near/3 (blocker? or antagonist?)):ti,ab |
| 23 | (Amlodipine or Amrinone or Bencyclane or Bepridil or Cinnarizine or Conotoxin? or Diltiazem or Felodipine or Fendiline or Flunarizine or Gallopamil or Isradipine or Lidoflazine or Mibefradil or Nicardipine or Nifedipine or Nimodipine or Nisoldipine or Nitrendipine or Perhexiline or Pregabalin or Prenylamine or Risedronate Sodium or Tiapamil Hydrochloride or Verapamil or omega-Agatoxin IVA or omega-Conotoxin?):ti,ab |
| 24 | Magnesium Sulfate:ti |
| 25 | MeSH descriptor: [DIURETICS] this term only |
| 26 | diuretic?:ti,ab |
| 27 | (Acetazolamide or Amiloride or Bendroflumethiazide or Bumetanide or Chlorothiazide or Chlorthalidone or Clopamide or Cyclopenthiazide or Ethacrynic Acid or Ethoxzolamide or Furosemide or Hydrochlorothiazide or Hydroflumethiazide or Indapamide or Mefruside or Methazolamide or Methyclothiazide or Metolazone or Muzolimine or Polythiazide or Potassium Citrate or Spironolactone or Ticrynafen or Triamterene or Trichlormethiazide or Xipamide or Isosorbide or Mannitol or Canrenoic Acid or Canrenone):ti,ab |
| 28 | MeSH descriptor: [ANGIOTENSIN-CONVERTING ENZYME INHIBITORS] 1 tree(s) exploded |
| 29 | (angiotensin converting enzyme near/3 (antagonist? or inhibitor?)):ti,ab |
| 30 | (ACE near/3 (antagonist? or inhibitor?)):ti,ab |
| 31 | (Captopril or Cilazapril or Enalapril or Enalaprilat or Fosinopril or Lisinopril or Perindopril or Ramipril or Teprotide):ti,ab |
| 32 | MeSH descriptor: [ASPIRIN] this term only |
| 33 | (acetylsalicylic acid or aspirin?):ti |
| 34 | ((elect* or plan*) near/3 deliver*):ti,ab |
| 35 | (expect* near/3 manag*):ti,ab |
| 36 | (tight* near/3 (manag* or control*)):ti,ab |
| 37 | MeSH descriptor: [BLOOD PRESSURE DETERMINATION] this term only |
| 38 | MeSH descriptor: [BLOOD PRESSURE MONITORING, AMBULATORY] this term only |
| 39 | ((Automat* or ambulatory or self*) near/3 blood pressure?):ti,ab |
| 40 | MeSH descriptor: [EXERCISE] 2 tree(s) exploded |
| 41 | exercis*:ti |
| 42 | (physical* activ* or swim* or cycl* or sport? or run* or jog* or walk* or stair climb* or gym* or resistance train* or yoga or pilates):ti,ab |
| 43 | MeSH descriptor: [DIET] 1 tree(s) exploded |
| 44 | diet*:ti |
| 45 | (calor* near/3 restrict*):ti,ab |
| 46 | ((portion? or serving) near/3 size?):ti,ab |
| 47 | ((low* or restrict*) near/3 (salt or sodium)):ti,ab |
| 48 | #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 |
| 49 | #12 and #48 |
Health economics search strategies
Databases: Medline; Medline EPub Ahead of Print; and Medline In-Process & Other Non-Indexed Citations
Date of last search: 19/02/18
| # | Searches |
|---|---|
| 1 | ECONOMICS/ |
| 2 | VALUE OF LIFE/ |
| 3 | exp “COSTS AND COST ANALYSIS”/ |
| 4 | exp ECONOMICS, HOSPITAL/ |
| 5 | exp ECONOMICS, MEDICAL/ |
| 6 | exp RESOURCE ALLOCATION/ |
| 7 | ECONOMICS, NURSING/ |
| 8 | ECONOMICS, PHARMACEUTICAL/ |
| 9 | exp “FEES AND CHARGES”/ |
| 10 | exp BUDGETS/ |
| 11 | budget*.ti,ab. |
| 12 | cost*.ti,ab. |
| 13 | (economic* or pharmaco?economic*).ti,ab. |
| 14 | (price* or pricing*).ti,ab. |
| 15 | (financ* or fee or fees or expenditure* or saving*).ti,ab. |
| 16 | (value adj2 (money or monetary)).ti,ab. |
| 17 | resourc* allocat*.ti,ab. |
| 18 | (fund or funds or funding* or funded).ti,ab. |
| 19 | (ration or rations or rationing* or rationed).ti,ab. |
| 20 | ec.fs. |
| 21 | or/1-20 |
| 22 | HYPERTENSION, PREGNANCY-INDUCED/ |
| 23 | PREGNANCY/ and HYPERTENSION/ |
| 24 | PRE-ECLAMPSIA/ |
| 25 | HELLP SYNDROME/ |
| 26 | ((pregnan$ or gestation$) adj5 hypertensi$).ti. |
| 27 | preeclamp$.ti,ab. |
| 28 | pre eclamp$.ti,ab. |
| 29 | HELLP.ti,ab. |
| 30 | tox?emi$.ti,ab. |
| 31 | or/22-30 |
| 32 | exp ADRENERGIC ALPHA-2 RECEPTOR AGONISTS/ |
| 33 | (alpha$ adj3 Agonist?).mp. |
| 34 | (Brimonidine Tartrate or Clonidine or exmedetomidine or Guanabenz or Guanfacine or Medetomidine or Methyldopa or Xylazine).mp. |
| 35 | exp ADRENERGIC BETA-ANTAGONISTS/ |
| 36 | (Adrenergic beta$ adj3 Antagonist?).mp. |
| 37 | Beta blocker?.mp. |
| 38 | (mixed adj3 blocker?).ti,ab. |
| 39 | (Alprenolol or Bunolol or Bupranolol or Carteolol or Dihydroalprenolol or Iodocyanopindolol or Labetalol or Levobunolol or Metipranolol or Nadolol or Oxprenolol or Penbutolol or Pindolol or Propranolol or Sotalol or Timolol or Acebutolol or Atenolol or Betaxolol or Bisoprolol or Celiprolol or Metoprolol or Practolol or Butoxamine).mp. |
| 40 | exp CALCIUM CHANNEL BLOCKERS/ |
| 41 | (calcium channel adj3 (blocker? or antagonist?)).ti,ab. |
| 42 | (Amlodipine or Amrinone or Bencyclane or Bepridil or Cinnarizine or Conotoxin? or Diltiazem or Felodipine or Fendiline or Flunarizine or Gallopamil or Isradipine or Lidoflazine or Mibefradil or Nicardipine or Nifedipine or Nimodipine or Nisoldipine or Nitrendipine or Perhexiline or Pregabalin or Prenylamine or Risedronate Sodium or Tiapamil Hydrochloride or Verapamil or omega-Agatoxin IVA or omega-Conotoxin?).mp. |
| 43 | Magnesium Sulfate.ti. |
| 44 | Magnesium Sulfate.ab. /freq=2 |
| 45 | DIURETICS/ |
| 46 | diuretic?.ti,ab. |
| 47 | (Acetazolamide or Amiloride or Bendroflumethiazide or Bumetanide or Chlorothiazide or Chlorthalidone or Clopamide or Cyclopenthiazide or Ethacrynic Acid or Ethoxzolamide or Furosemide or Hydrochlorothiazide or Hydroflumethiazide or Indapamide or Mefruside or Methazolamide or Methyclothiazide or Metolazone or Muzolimine or Polythiazide or Potassium Citrate or Spironolactone or Ticrynafen or Triamterene or Trichlormethiazide or Xipamide or Isosorbide or Mannitol or Canrenoic Acid or Canrenone).mp. |
| 48 | exp ANGIOTENSIN-CONVERTING ENZYME INHIBITORS/ |
| 49 | (angiotensin converting enzyme adj3 (antagonist? or inhibitor?)).ti,ab. |
| 50 | (ACE adj3 (antagonist? or inhibitor?)).ti,ab. |
| 51 | (Captopril or Cilazapril or Enalapril or Enalaprilat or Fosinopril or Lisinopril or Perindopril or Ramipril or Teprotide).mp. |
| 52 | ASPIRIN/ |
| 53 | (acetylsalicylic acid or aspirin?).ti. |
| 54 | (acetylsalicylic acid or aspirin?).ab. /freq=2 |
| 55 | ((elect$ or plan$) adj3 deliver$).ti,ab. |
| 56 | (expect$ adj3 manag$).ti,ab. |
| 57 | (tight$ adj3 (manag$ or control$)).ti,ab. |
| 58 | BLOOD PRESSURE DETERMINATION/ |
| 59 | BLOOD PRESSURE MONITORING, AMBULATORY/ |
| 60 | ((Automat$ or ambulatory or self$) adj3 blood pressure?).ti,ab. |
| 61 | exp EXERCISE/ |
| 62 | (exercis$ or physical$ activ$ or swim$ or cycl$ or sport? or run$ or jog$ or walk$ or stair climb$ or gym$ or resistance train$ or yoga or pilates).ti. |
| 63 | (exercis$ or physical$ activ$ or swim$ or cycl$ or sport? or run$ or jog$ or walk$ or stair climb$ or gym$ or resistance train$ or yoga or pilates).ab. /freq=2 |
| 64 | exp DIET/ |
| 65 | diet$.ti. |
| 66 | diet$.ab. /freq=2 |
| 67 | (calor$ adj3 restrict$).ti,ab. |
| 68 | ((portion? or serving) adj3 size?).ti,ab. |
| 69 | ((low$ or restrict$) adj3 (salt or sodium)).ti,ab. |
| 70 | or/32-69 |
| 71 | 31 and 70 |
| 72 | limit 71 to english language |
| 73 | LETTER/ |
| 74 | EDITORIAL/ |
| 75 | NEWS/ |
| 76 | exp HISTORICAL ARTICLE/ |
| 77 | ANECDOTES AS TOPIC/ |
| 78 | COMMENT/ |
| 79 | CASE REPORT/ |
| 80 | (letter or comment*).ti. |
| 81 | or/73-80 |
| 82 | RANDOMIZED CONTROLLED TRIAL/ or random*.ti,ab. |
| 83 | 81 not 82 |
| 84 | ANIMALS/ not HUMANS/ |
| 85 | exp ANIMALS, LABORATORY/ |
| 86 | exp ANIMAL EXPERIMENTATION/ |
| 87 | exp MODELS, ANIMAL/ |
| 88 | exp RODENTIA/ |
| 89 | (rat or rats or mouse or mice).ti. |
| 90 | or/83-89 |
| 91 | 72 not 90 |
| 92 | 21 and 91 |
Databases: Embase; and Embase Classic
Date of last search: 19/02/18
| # | Searches |
|---|---|
| 1 | HEALTH ECONOMICS/ |
| 2 | exp ECONOMIC EVALUATION/ |
| 3 | exp HEALTH CARE COST/ |
| 4 | exp FEE/ |
| 5 | BUDGET/ |
| 6 | FUNDING/ |
| 7 | RESOURCE ALLOCATION/ |
| 8 | budget*.ti,ab. |
| 9 | cost*.ti,ab. |
| 10 | (economic* or pharmaco?economic*).ti,ab. |
| 11 | (price* or pricing*).ti,ab. |
| 12 | (financ* or fee or fees or expenditure* or saving*).ti,ab. |
| 13 | (value adj2 (money or monetary)).ti,ab. |
| 14 | resourc* allocat*.ti,ab. |
| 15 | (fund or funds or funding* or funded).ti,ab. |
| 16 | (ration or rations or rationing* or rationed).ti,ab. |
| 17 | or/1-16 |
| 18 | MATERNAL HYPERTENSION/ |
| 19 | PREGNANCY/ and HYPERTENSION/ |
| 20 | PREECLAMPSIA/ |
| 21 | HELLP SYNDROME/ |
| 22 | ((pregnan$ or gestation$) adj5 hypertensi$).ti. |
| 23 | preeclamp$.ti,ab. |
| 24 | pre eclamp$.ti,ab. |
| 25 | HELLP.ti,ab. |
| 26 | tox?emi$.ti,ab. |
| 27 | or/18-26 |
| 28 | exp *ALPHA 2 ADRENERGIC RECEPTOR STIMULATING AGENT/ |
| 29 | (alpha$ adj3 Agonist?).mp. |
| 30 | (Brimonidine Tartrate or Clonidine or exmedetomidine or Guanabenz or Guanfacine or Medetomidine or Methyldopa or Xylazine).mp. |
| 31 | exp *BETA ADRENERGIC RECEPTOR BLOCKING AGENT/ |
| 32 | (adrenergic adj3 beta adj3 antagonist?).ti,ab. |
| 33 | Beta blocker?.mp. |
| 34 | (mixed adj3 blocker?).ti,ab. |
| 35 | (Alprenolol or Bunolol or Bupranolol or Carteolol or Dihydroalprenolol or Iodocyanopindolol or Labetalol or Levobunolol or Metipranolol or Nadolol or Oxprenolol or Penbutolol or Pindolol or Propranolol or Sotalol or Timolol or Acebutolol or Atenolol or Betaxolol or Bisoprolol or Celiprolol or Metoprolol or Practolol or Butoxamine).mp. |
| 36 | exp *CALCIUM CHANNEL BLOCKING AGENT/ |
| 37 | (calcium channel adj3 (blocker? or antagonist?)).ti,ab. |
| 38 | (Amlodipine or Amrinone or Bencyclane or Bepridil or Cinnarizine or Conotoxin? or Diltiazem or Felodipine or Fendiline or Flunarizine or Gallopamil or Isradipine or Lidoflazine or Mibefradil or Nicardipine or Nifedipine or Nimodipine or Nisoldipine or Nitrendipine or Perhexiline or Pregabalin or Prenylamine or Risedronate Sodium or Tiapamil Hydrochloride or Verapamil or omega-Agatoxin IVA or omega-Conotoxin?).mp. |
| 39 | Magnesium Sulfate.ti. |
| 40 | Magnesium Sulfate.ab. /freq=2 |
| 41 | exp *DIURETIC AGENT/ |
| 42 | diuretic?.ti,ab. |
| 43 | (Acetazolamide or Amiloride or Bendroflumethiazide or Bumetanide or Chlorothiazide or Chlorthalidone or Clopamide or Cyclopenthiazide or Ethacrynic Acid or Ethoxzolamide or Furosemide or Hydrochlorothiazide or Hydroflumethiazide or Indapamide or Mefruside or Methazolamide or Methyclothiazide or Metolazone or Muzolimine or Polythiazide or Potassium Citrate or Spironolactone or Ticrynafen or Triamterene or Trichlormethiazide or Xipamide or Isosorbide or Mannitol or Canrenoic Acid or Canrenone).mp. |
| 44 | exp *DIPEPTIDYL CARBOXYPEPTIDASE INHIBITOR/ |
| 45 | (angiotensin converting enzyme adj3 (antagonist? or inhibitor?)).ti,ab. |
| 46 | (ACE adj3 (antagonist? or inhibitor?)).ti,ab. |
| 47 | (Captopril or Cilazapril or Enalapril or Enalaprilat or Fosinopril or Lisinopril or Perindopril or Ramipril or Teprotide).mp. |
| 48 | *ACETYLSALICYLIC ACID/ |
| 49 | (acetylsalicylic acid or aspirin?).ti. |
| 50 | (acetylsalicylic acid or aspirin?).ab. /freq=2 |
| 51 | ((elect$ or plan$) adj3 deliver$).ti,ab. |
| 52 | (expect$ adj3 manag$).ti,ab. |
| 53 | (tight$ adj3 (manag$ or control$)).ti,ab. |
| 54 | *BLOOD PRESSURE MEASUREMENT/ |
| 55 | *BLOOD PRESSURE MONITORING/ |
| 56 | ((Automat$ or ambulatory or self$) adj3 blood pressure?).ti,ab. |
| 57 | exp *EXERCISE/ |
| 58 | (exercis$ or physical$ activ$ or swim$ or cycl$ or sport? or run$ or jog$ or walk$ or stair climb$ or gym$ or resistance train$ or yoga or pilates).ti. |
| 59 | (exercis$ or physical$ activ$ or swim$ or cycl$ or sport? or run$ or jog$ or walk$ or stair climb$ or gym$ or resistance train$ or yoga or pilates).ab. /freq=2 |
| 60 | exp *DIET/ |
| 61 | diet$.ti. |
| 62 | diet$.ab. /freq=2 |
| 63 | (calor$ adj3 restrict$).ti,ab. |
| 64 | ((portion? or serving) adj3 size?).ti,ab. |
| 65 | *SODIUM RESTRICTION/ |
| 66 | ((low$ or restrict$) adj3 (salt or sodium)).ti,ab. |
| 67 | or/28-66 |
| 68 | 27 and 67 |
| 69 | limit 68 to english language |
| 70 | letter.pt. or LETTER/ |
| 71 | note.pt. |
| 72 | editorial.pt. |
| 73 | CASE REPORT/ or CASE STUDY/ |
| 74 | (letter or comment*).ti. |
| 75 | or/70-74 |
| 76 | RANDOMIZED CONTROLLED TRIAL/ or random*.ti,ab. |
| 77 | 75 not 76 |
| 78 | ANIMAL/ not HUMAN/ |
| 79 | NONHUMAN/ |
| 80 | exp ANIMAL EXPERIMENT/ |
| 81 | exp EXPERIMENTAL ANIMAL/ |
| 82 | ANIMAL MODEL/ |
| 83 | exp RODENT/ |
| 84 | (rat or rats or mouse or mice).ti. |
| 85 | or/77-84 |
| 86 | 69 not 85 |
| 87 | 17 and 86 |
Database: Cochrane Central Register of Controlled Trials
Date of last search: 19/02/18
| # | Searches |
|---|---|
| 1 | MeSH descriptor: [ECONOMICS] this term only |
| 2 | MeSH descriptor: [VALUE OF LIFE] this term only |
| 3 | MeSH descriptor: [COSTS AND COST ANALYSIS] explode all trees |
| 4 | MeSH descriptor: [ECONOMICS, HOSPITAL] explode all trees |
| 5 | MeSH descriptor: [ECONOMICS, MEDICAL] explode all trees |
| 6 | MeSH descriptor: [RESOURCE ALLOCATION] explode all trees |
| 7 | MeSH descriptor: [ECONOMICS, NURSING] this term only |
| 8 | MeSH descriptor: [ECONOMICS, PHARMACEUTICAL] this term only |
| 9 | MeSH descriptor: [FEES AND CHARGES] explode all trees |
| 10 | MeSH descriptor: [BUDGETS] explode all trees |
| 11 | budget*:ti,ab |
| 12 | cost*:ti,ab |
| 13 | (economic* or pharmaco?economic*):ti,ab |
| 14 | (price* or pricing*):ti,ab |
| 15 | (financ* or fee or fees or expenditure* or saving*):ti,ab |
| 16 | (value near/2 (money or monetary)):ti,ab |
| 17 | resourc* allocat*:ti,ab |
| 18 | (fund or funds or funding* or funded):ti,ab |
| 19 | (ration or rations or rationing* or rationed):ti,ab |
| 20 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 |
| 21 | MeSH descriptor: [HYPERTENSION, PREGNANCY-INDUCED] this term only |
| 22 | MeSH descriptor: [PREGNANCY] this term only |
| 23 | MeSH descriptor: [HYPERTENSION] this term only |
| 24 | #2 and #3 |
| 25 | MeSH descriptor: [PRE-ECLAMPSIA] this term only |
| 26 | MeSH descriptor: [HELLP SYNDROME] this term only |
| 27 | ((pregnan* or gestation*) near/5 hypertensi*):ti |
| 28 | preeclamp*:ti,ab |
| 29 | pre eclamp*:ti,ab |
| 30 | HELLP:ti,ab |
| 31 | tox?emi*:ti,ab |
| 32 | #21 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 |
| 33 | MeSH descriptor: [ADRENERGIC ALPHA-2 RECEPTOR AGONISTS] 2 tree(s) exploded |
| 34 | (alpha* near/3 Agonist?):ti,ab |
| 35 | (Brimonidine Tartrate or Clonidine or exmedetomidine or Guanabenz or Guanfacine or Medetomidine or Methyldopa or Xylazine):ti,ab |
| 36 | MeSH descriptor: [ADRENERGIC BETA-ANTAGONISTS] 2 tree(s) exploded |
| 37 | (Adrenergic beta* near/3 Antagonist?):ti,ab |
| 38 | Beta blocker?:ti,ab |
| 39 | (mixed near/3 blocker?):ti,ab |
| 40 | (Alprenolol or Bunolol or Bupranolol or Carteolol or Dihydroalprenolol or Iodocyanopindolol or Labetalol or Levobunolol or Metipranolol or Nadolol or Oxprenolol or Penbutolol or Pindolol or Propranolol or Sotalol or Timolol or Acebutolol or Atenolol or Betaxolol or Bisoprolol or Celiprolol or Metoprolol or Practolol or Butoxamine):ti,ab |
| 41 | MeSH descriptor: [CALCIUM CHANNEL BLOCKERS] 2 tree(s) exploded |
| 42 | (calcium channel near/3 (blocker? or antagonist?)):ti,ab |
| 43 | (Amlodipine or Amrinone or Bencyclane or Bepridil or Cinnarizine or Conotoxin? or Diltiazem or Felodipine or Fendiline or Flunarizine or Gallopamil or Isradipine or Lidoflazine or Mibefradil or Nicardipine or Nifedipine or Nimodipine or Nisoldipine or Nitrendipine or Perhexiline or Pregabalin or Prenylamine or Risedronate Sodium or Tiapamil Hydrochloride or Verapamil or omega-Agatoxin IVA or omega-Conotoxin?):ti,ab |
| 44 | Magnesium Sulfate:ti |
| 45 | MeSH descriptor: [DIURETICS] this term only |
| 46 | diuretic?:ti,ab |
| 47 | (Acetazolamide or Amiloride or Bendroflumethiazide or Bumetanide or Chlorothiazide or Chlorthalidone or Clopamide or Cyclopenthiazide or Ethacrynic Acid or Ethoxzolamide or Furosemide or Hydrochlorothiazide or Hydroflumethiazide or Indapamide or Mefruside or Methazolamide or Methyclothiazide or Metolazone or Muzolimine or Polythiazide or Potassium Citrate or Spironolactone or Ticrynafen or Triamterene or Trichlormethiazide or Xipamide or Isosorbide or Mannitol or Canrenoic Acid or Canrenone):ti,ab |
| 48 | MeSH descriptor: [ANGIOTENSIN-CONVERTING ENZYME INHIBITORS] 1 tree(s) exploded |
| 49 | (angiotensin converting enzyme near/3 (antagonist? or inhibitor?)):ti,ab |
| 50 | (ACE near/3 (antagonist? or inhibitor?)):ti,ab |
| 51 | (Captopril or Cilazapril or Enalapril or Enalaprilat or Fosinopril or Lisinopril or Perindopril or Ramipril or Teprotide):ti,ab |
| 52 | MeSH descriptor: [ASPIRIN] this term only |
| 53 | (acetylsalicylic acid or aspirin?):ti |
| 54 | ((elect* or plan*) near/3 deliver*):ti,ab |
| 55 | (expect* near/3 manag*):ti,ab |
| 56 | (tight* near/3 (manag* or control*)):ti,ab |
| 57 | MeSH descriptor: [BLOOD PRESSURE DETERMINATION] this term only |
| 58 | MeSH descriptor: [BLOOD PRESSURE MONITORING, AMBULATORY] this term only |
| 59 | ((Automat* or ambulatory or self*) near/3 blood pressure?):ti,ab |
| 60 | MeSH descriptor: [EXERCISE] 2 tree(s) exploded |
| 61 | exercis*:ti |
| 62 | (physical* activ* or swim* or cycl* or sport? or run* or jog* or walk* or stair climb* or gym* or resistance train* or yoga or pilates):ti,ab |
| 63 | MeSH descriptor: [DIET] 1 tree(s) exploded |
| 64 | diet*:ti |
| 65 | (calor* near/3 restrict*):ti,ab |
| 66 | ((portion? or serving) near/3 size?):ti,ab |
| 67 | ((low* or restrict*) near/3 (salt or sodium)):ti,ab |
| 68 | #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 |
| 69 | #12 and #48 |
| 70 | #20 and #69 |
Databases: Health Technology Assessment; and NHS Economic Evaluation Database
Date of last search: 19/02/18
| # | Searches |
|---|---|
| 1 | MeSH descriptor: [HYPERTENSION, PREGNANCY-INDUCED] this term only |
| 2 | MeSH descriptor: [PREGNANCY] this term only |
| 3 | MeSH descriptor: [HYPERTENSION] this term only |
| 4 | #2 and #3 |
| 5 | MeSH descriptor: [PRE-ECLAMPSIA] this term only |
| 6 | MeSH descriptor: [HELLP SYNDROME] this term only |
| 7 | ((pregnan* or gestation*) near/5 hypertensi*):ti |
| 8 | preeclamp*:ti,ab |
| 9 | pre eclamp*:ti,ab |
| 10 | HELLP:ti,ab |
| 11 | tox?emi*:ti,ab |
| 12 | #1 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 |
| 13 | MeSH descriptor: [ADRENERGIC ALPHA-2 RECEPTOR AGONISTS] 2 tree(s) exploded |
| 14 | (alpha* near/3 Agonist?):ti,ab |
| 15 | (Brimonidine Tartrate or Clonidine or exmedetomidine or Guanabenz or Guanfacine or Medetomidine or Methyldopa or Xylazine):ti,ab |
| 16 | MeSH descriptor: [ADRENERGIC BETA-ANTAGONISTS] 2 tree(s) exploded |
| 17 | (Adrenergic beta* near/3 Antagonist?):ti,ab |
| 18 | Beta blocker?:ti,ab |
| 19 | (mixed near/3 blocker?):ti,ab |
| 20 | (Alprenolol or Bunolol or Bupranolol or Carteolol or Dihydroalprenolol or Iodocyanopindolol or Labetalol or Levobunolol or Metipranolol or Nadolol or Oxprenolol or Penbutolol or Pindolol or Propranolol or Sotalol or Timolol or Acebutolol or Atenolol or Betaxolol or Bisoprolol or Celiprolol or Metoprolol or Practolol or Butoxamine):ti,ab |
| 21 | MeSH descriptor: [CALCIUM CHANNEL BLOCKERS] 2 tree(s) exploded |
| 22 | (calcium channel near/3 (blocker? or antagonist?)):ti,ab |
| 23 | (Amlodipine or Amrinone or Bencyclane or Bepridil or Cinnarizine or Conotoxin? or Diltiazem or Felodipine or Fendiline or Flunarizine or Gallopamil or Isradipine or Lidoflazine or Mibefradil or Nicardipine or Nifedipine or Nimodipine or Nisoldipine or Nitrendipine or Perhexiline or Pregabalin or Prenylamine or Risedronate Sodium or Tiapamil Hydrochloride or Verapamil or omega-Agatoxin IVA or omega-Conotoxin?):ti,ab |
| 24 | Magnesium Sulfate:ti |
| 25 | MeSH descriptor: [DIURETICS] this term only |
| 26 | diuretic?:ti,ab |
| 27 | (Acetazolamide or Amiloride or Bendroflumethiazide or Bumetanide or Chlorothiazide or Chlorthalidone or Clopamide or Cyclopenthiazide or Ethacrynic Acid or Ethoxzolamide or Furosemide or Hydrochlorothiazide or Hydroflumethiazide or Indapamide or Mefruside or Methazolamide or Methyclothiazide or Metolazone or Muzolimine or Polythiazide or Potassium Citrate or Spironolactone or Ticrynafen or Triamterene or Trichlormethiazide or Xipamide or Isosorbide or Mannitol or Canrenoic Acid or Canrenone):ti,ab |
| 28 | MeSH descriptor: [ANGIOTENSIN-CONVERTING ENZYME INHIBITORS] 1 tree(s) exploded |
| 29 | (angiotensin converting enzyme near/3 (antagonist? or inhibitor?)):ti,ab |
| 30 | (ACE near/3 (antagonist? or inhibitor?)):ti,ab |
| 31 | (Captopril or Cilazapril or Enalapril or Enalaprilat or Fosinopril or Lisinopril or Perindopril or Ramipril or Teprotide):ti,ab |
| 32 | MeSH descriptor: [ASPIRIN] this term only |
| 33 | (acetylsalicylic acid or aspirin?):ti |
| 34 | ((elect* or plan*) near/3 deliver*):ti,ab |
| 35 | (expect* near/3 manag*):ti,ab |
| 36 | (tight* near/3 (manag* or control*)):ti,ab |
| 37 | MeSH descriptor: [BLOOD PRESSURE DETERMINATION] this term only |
| 38 | MeSH descriptor: [BLOOD PRESSURE MONITORING, AMBULATORY] this term only |
| 39 | ((Automat* or ambulatory or self*) near/3 blood pressure?):ti,ab |
| 40 | MeSH descriptor: [EXERCISE] 2 tree(s) exploded |
| 41 | exercis*:ti |
| 42 | (physical* activ* or swim* or cycl* or sport? or run* or jog* or walk* or stair climb* or gym* or resistance train* or yoga or pilates):ti,ab |
| 43 | MeSH descriptor: [DIET] 1 tree(s) exploded |
| 44 | diet*:ti |
| 45 | (calor* near/3 restrict*):ti,ab |
| 46 | ((portion? or serving) near/3 size?):ti,ab |
| 47 | ((low* or restrict*) near/3 (salt or sodium)):ti,ab |
| 48 | #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 |
| 49 | #12 and #48 |
Appendix D. Clinical evidence tables
Table 4. Clinical evidence tables (PDF, 1.0M)
Appendix E. Forest plots
No forest plots were generated for comparisons 1-6 and 8-10, as no meta-analyses were performed
Appendix F. GRADE tables
Table 5Clinical evidence profile. Comparison 1. Induction of labour versus expectant management
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Induction of labour | Expectant management | Relative (95% CI) | Absolute | ||
| Perinatal mortality | ||||||||||||
| 1 (Hamed 2014) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
2/38 (5.3%) |
1/38 (2.6%) | RR 2 (0.19 to 21.14) | 26 more per 1000 (from 21 fewer to 530 more) | VERY LOW | CRITICAL |
| Birth weight (grams) (Better indicated by higher values) | ||||||||||||
| 1 (Hamed 2014) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none | 38 | 38 | - | MD 400 lower (669.79 to 130.21 lower) | LOW | IMPORTANT |
| Gestational age at birth (weeks) (Better indicated by higher values) | ||||||||||||
| 1 (Hamed 2014) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | serious3 | none | 38 | 38 | - | MD 2.40 lower (3.34 to 1.46 lower) | VERY LOW | IMPORTANT |
| Preterm birth (number of weeks were not reported) | ||||||||||||
| 1 (Hamed 2014) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
10/38 (26.3%) |
12/38 (31.6%) | RR 0.83 (0.41 to 1.69) | 54 fewer per 1000 (from 186 fewer to 218 more) | VERY LOW | IMPORTANT |
| Admission to neonatal unit | ||||||||||||
| 1 (Hamed 2014) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | serious4 | none |
12/38 (31.6%) |
3/38 (7.9%) | RR 4.00 (1.23 to 13.05) | 237 more per 1000 (from 18 more to 951 more) | VERY LOW | IMPORTANT |
| Severe hypertension | ||||||||||||
| 1 (Hamed 2014) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
5/38 (13.2%) |
3/38 (7.9%) | RR 1.67 (0.43 to 6.49) | 53 more per 1000 (from 45 fewer to 433 more) | VERY LOW | CRITICAL |
| Superimposed pre-eclampsia | ||||||||||||
| 1 (Hamed 2014) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
12/38 (31.6%) |
13/38 (34.2%) | RR 0.92 (0.49 to 1.76) | 27 fewer per 1000 (from 174 fewer to 260 more) | VERY LOW | IMPORTANT |
| Placental abruption | ||||||||||||
| 1 (Hamed 2014) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
3/38 (7.9%) |
3/38 (7.9%) | RR 1.00 (0.22 to 4.65) | 0 fewer per 1000 (from 62 fewer to 288 more) | VERY LOW | IMPORTANT |
- 1
The quality of the evidence was downgraded by two levels due to unclear risk of allocation concealment, performance and selection bias, and selective reporting
- 2
The quality of the evidence was downgraded by two levels as the 95% CI crossed 2 default MID thresholds (0.8 and 1.25)
- 3
The quality of the evidence was downgraded by one level as the 95% CI crossed 1 MID threshold (3.9 × +/− 0.5 = +/− 1.95)
- 4
The quality of the evidence was downgraded by one level as the 95% CI crossed 1 default MID threshold (1.25)
Table 6Clinical evidence profile. Comparison 2. Exercise versus no intervention
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Exercise | No intervention | Relative (95% CI) | Absolute | ||
| Birth weight <2500 grams | ||||||||||||
| 1 (Kasawara 2013) | randomised trials | very serious1 | no serious inconsistency | serious2 | very serious3 | none |
9/56 (16.1%) |
11/53 (20.8%) | RR 0.77 (0.35 to 1.72) | 48 fewer per 1000 (from 135 fewer to 149 more) | VERY LOW | IMPORTANT |
| Birth weight 2500-3999 grams | ||||||||||||
| 1 (Kasawara 2013) | randomised trials | very serious1 | no serious inconsistency | serious2 | serious4 | none |
41/56 (73.2%) |
35/53 (66%) | RR 1.11 (0.86 to 1.42) | 73 more per 1000 (from 92 fewer to 277 more) | VERY LOW | IMPORTANT |
| Birth weight ≥4000 grams | ||||||||||||
| 1 (Kasawara 2013) | randomised trials | very serious1 | no serious inconsistency | serious2 | serious5 | none |
5/56 (8.9%) |
11/53 (20.8%) | RR 0.43 (0.16 to 1.16) | 118 fewer per 1000 (from 174 fewer to 33 more) | VERY LOW | IMPORTANT |
| Admission to neonatal unit | ||||||||||||
| 1 (Kasawara 2013) | randomised trials | very serious1 | no serious inconsistency | serious2 | very serious3 | none |
12/56 (21.4%) |
13/53 (24.5%) | RR 0.87 (0.44 to 1.74) | 32 fewer per 1000 (from 137 fewer to 182 more) | VERY LOW | IMPORTANT |
| Mode of birth (caesarean section) | ||||||||||||
| 1 (Kasawara 2013) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | serious5 | none |
36/56 (64.3%) |
41/53 (77.4%) | RR 0.83 (0.65 to 1.06) | 132 fewer per 1000 (from 271 fewer to 46 more) | VERY LOW | IMPORTANT |
- 1
The quality of the evidence was downgraded by 2 levels as participants and personnel were not blinded to treatment allocation; it was unclear whether outcome assessors were blinded to treatment allocation and there was an unclear risk of selective reporting
- 2
The quality of the evidence was downgraded by 1 level as 9.49% of women did not present with chronic hypertension
- 3
The quality of the evidence was downgraded by 2 levels as the 95% CI crossed 2 default MID thresholds (0.8 and 1.25)
- 4
The quality of the evidence was downgraded by 1 level as the 95% CI crossed 1 default MID threshold (1.25)
- 5
The quality of the evidence was downgraded by 1 level as the 95% CI crossed 1 default MID threshold (0.8)
Table 7Clinical evidence profile. Comparison 3. Less-tight versus tight control of blood pressure
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Less-tight control | Tight control | Relative (95% CI) | Absolute | ||
| Stillbirth | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | serious2 | very serious3 | none |
12/493 (2.4%) |
7/488 (1.4%) | RR 1.70 (0.67 to 4.27) | 10 more per 1000 (from 5 fewer to 47 more) | VERY LOW | CRITICAL |
| Neonatal death | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | serious2 | very serious3 | none |
2/493 (0.41%) |
4/488 (0.82%) | RR 0.49 (0.09 to 2.69) | 4 fewer per 1000 (from 7 fewer to 14 more) | VERY LOW | CRITICAL |
| Small for gestational age (birthweight <10th percentile) | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | serious4 | none |
51/366 (13.9%) |
71/361 (19.7%) | RR 0.71 (0.51 to 0.98) | 57 fewer per 1000 (from 4 fewer to 96 fewer) | LOW | CRITICAL |
| Birth weight (grams) (Better indicated by higher values) | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | serious2 | no serious imprecision | none | 493 | 488 | - | MD 31.07 lower (66.68 lower to 4.54 higher) | LOW | IMPORTANT |
| Gestational age at delivery (weeks) (Better indicated by higher values) | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | serious2 | no serious imprecision | none | 493 | 488 | - | MD 0.40 lower (0.81 lower to 0.01 higher) | LOW | IMPORTANT |
| Admission to neonatal unit | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | serious2 | no serious imprecision | none |
141/480 (29.4%) |
139/479 (29%) | RR 1.01 (0.83 to 1.23) | 3 more per 1000 (from 49 fewer to 67 more) | LOW | IMPORTANT |
| Severe hypertension | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none |
159/369 (43.1%) |
96/363 (26.4%) | RR 1.63 (1.32 to 2.01) | 167 more per 1000 (from 85 more to 267 more) | MODERATE | CRITICAL |
| HELLP | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious2 | no serious inconsistency | serious2 | very serious3 | none |
9/493 (1.8%) |
2/488 (0.41%) | RR 4.45 (0.97 to 20.51) | 14139 more per 1,000,000 (from 123 fewer to 79959 more) | VERY LOW | IMPORTANT |
| Placental abruption | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | serious2 | very serious3 | none |
11/493 (2.2%) |
11/488 (2.3%) | RR 0.99 (0.43 to 2.26) | 0 fewer per 1000 (from 13 fewer to 28 more) | VERY LOW | IMPORTANT |
| Pre-eclampsia | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | serious5 | none |
176/368 (47.8%) |
155/363 (42.7%) | RR 1.12 (0.95 to 1.31) | 51 more per 1000 (from 21 fewer to 132 more) | LOW | IMPORTANT |
| Onset of labour (spontaneous onset) | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | serious2 | serious5 | none |
109/493 (22.1%) |
104/488 (21.3%) | RR 1.04 (0.82 to 1.32) | 9 more per 1000 (from 38 fewer to 68 more) | VERY LOW | IMPORTANT |
| Onset of labour (induced) | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | serious2 | no serious imprecision | none |
224/493 (45.4%) |
218/488 (44.7%) | RR 1.02 (0.89 to 1.17) | 9 more per 1000 (from 49 fewer to 76 more) | LOW | IMPORTANT |
| Onset of labour (elective caesarean section) | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | serious2 | no serious imprecision | none |
159/493 (32.3%) |
164/488 (33.6%) | RR 0.96 (0.80 to 1.15) | 13 fewer per 1000 (from 67 fewer to 50 more) | LOW | IMPORTANT |
| Mode of birth (C-section) | ||||||||||||
| 1 (Magee 2015) | randomised trials | serious1 | no serious inconsistency | serious2 | no serious imprecision | none |
231/493 (46.9%) |
250/488 (51.2%) | RR 0.91 (0.80 to 1.04) | 46 fewer per 1000 (from 102 fewer to 20 more) | LOW | IMPORTANT |
- 1
The quality of the evidence was downgraded by 1 level due to a high risk of performance and detection bias
- 2
The quality of the evidence was downgraded by 1 level as 25.5% of women did not present with chronic hypertension
- 3
The quality of the evidence was downgraded by 2 levels as the 95% CI crossed 2 default MID thresholds (0.8 and 1.25)
- 4
The quality of the evidence was downgraded by 1 level as the 95% CI crossed 1 default MID threshold (0.8)
- 5
The quality of the evidence was downgraded by 1 level as the 95% CI crossed 1 default MID threshold (1.25)
Table 8Clinical evidence profile. Comparison 4. Atenolol versus placebo
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Atenolol | Placebo | Relative (95% CI) | Absolute | ||
| Stillbirth | ||||||||||||
| 1 (Butters 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
1/15 (6.7%) |
0/14 (0%) | RR 2.81 (0.12 to 63.83)5 | - | VERY LOW | CRITICAL |
| Small-for-gestational age | ||||||||||||
| 1 (Butters 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none |
10/15 (66.7%) |
0/14 (0%) | RR 19.69 (1.26 to 307.41)5 | - | LOW | CRITICAL |
| Birth weight (grams) (Better indicated by higher values) | ||||||||||||
| 1 (Butters 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious3 | none | 15 | 14 | - | MD 910 lower (440 to 1380) | VERY LOW | IMPORTANT |
| Gestational age at birth (weeks) (Better indicated by higher values) | ||||||||||||
| 1 (Butters 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious3 | none | 15 | 14 | - | not calculable4 | VERY LOW | IMPORTANT |
| sBP after treatment | ||||||||||||
| 1 (Butters 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious3 | none | - | - | - | MD 4 higher (1.4 lower to 8.6 higher) | VERY LOW | IMPORTANT |
| dBP after treatment | ||||||||||||
| 1 (Butters 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious3 | none | - | - | - | MD 7 lower (2.9 to 10 lower) | VERY LOW | IMPORTANT |
- 1
The quality of the evidence was downgraded by 2 levels due to an unclear risk of random sequence generation and allocation concealment and a high risk of selective reporting
- 2
The quality of the evidence was downgraded by 2 levels as the 95% CI crossed 2 default MID thresholds (0.8 and 1.25)
- 3
The quality of the evidence was downgraded by 2 levels as imprecision could not be assessed as SDs have not been reported
- 4
Not enough information was provided to allow calculation (SDs have not been reported). The mean gestational age in the atenolol group was 39.5 weeks and in the placebo group was 38.5 weeks
- 5
Corresponding absolute risk was not calculated as there were no events reported in the control arm.
Table 9Clinical evidence profile. Comparison 5. Labetalol versus no intervention
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Labetalol | No intervention | Relative (95% CI) | Absolute | ||
| Perinatal death | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
1/86 (1.2%) |
1/90 (1.1%) | RR 1.05 (0.07 to 16.47) | 1 more per 1000 (from 10 fewer to 172 more) | VERY LOW | CRITICAL |
| Small-for-gestational age | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
7/86 (8.1%) |
8/90 (8.9%) | RR 0.92 (0.35 to 2.42) | 7 fewer per 1000 (from 58 fewer to 126 more) | VERY LOW | CRITICAL |
| Preterm birth (<37 weeks) | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
10/86 (11.6%) |
9/90 (10%) | RR 1.16 (0.50 to 2.72) | 16 more per 1000 (from 50 fewer to 172 more) | VERY LOW | IMPORTANT |
| Superimposed pre-eclampsia | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
14/86 (16.3%) |
14/90 (15.6%) | RR 1.05 (0.53 to 2.06) | 8 more per 1000 (from 73 fewer to 165 more) | VERY LOW | IMPORTANT |
| Placental abruption | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
2/86 (2.3%) |
2/90 (2.2%) | RR 1.05 (0.15 to 7.26) | 1 more per 1000 (from 19 fewer to 139 more) | VERY LOW | IMPORTANT |
| Mode of birth (caesarean section) | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
30/86 (34.9%) |
29/90 (32.2%) | RR 1.08 (0.71 to 1.64) | 26 more per 1000 (from 93 fewer to 206 more) | VERY LOW | IMPORTANT |
- 1
The quality of the evidence was downgraded by 2 levels due to an unclear risk of allocation concealment, performance and selection bias, and selective reporting
- 2
The quality of the evidence was downgraded by 2 levels as the 95% CI crossed 2 default MID thresholds (0.8 and 1.25)
Table 10Clinical evidence profile. Comparison 6. Labetalol versus nifedipine
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Labetalol | Nifedipine | Relative (95% CI) | Absolute | ||
| Stillbirth | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
2/55 (3.6%) |
1/57 (1.8%) | RR 2.07 (0.19 to 22.21) | 19 more per 1000 (from 14 fewer to 372 more) | VERY LOW | CRITICAL |
| Neonatal death | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none |
0/55 (0%) |
0/57 (0%) | not calculable | not calculable | MODERATE | CRITICAL |
| Small-for-gestational age | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
16/55 (29.1%) |
17/57 (29.8%) | RR 0.98 (0.55 to 1.73) | 6 fewer per 1000 (from 134 fewer to 218 more) | VERY LOW | CRITICAL |
| Birth weight (grams) (Better indicated by higher values) | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | serious3 | none | 55 | 57 | - | MD 225 higher (85.06 lower to 535.06 higher) | LOW | IMPORTANT |
| Preterm birth (<37 weeks) | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | serious4 | none |
12/55 (21.8%) |
20/57 (35.1%) | RR 0.62 (0.34 to 1.15) | 133 fewer per 1000 (from 232 fewer to 53 more) | LOW | IMPORTANT |
| Preterm birth (<34 weeks) | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
10/55 (18.2%) |
11/57 (19.3%) | RR 0.94 (0.44 to 2.04) | 12 fewer per 1000 (from 108 fewer to 201 more) | VERY LOW | IMPORTANT |
| Admission to neonatal unit | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
11/55 (20%) |
15/57 (26.3%) | RR 0.76 (0.38 to 1.51) | 63 fewer per 1000 (from 163 fewer to 134 more) | VERY LOW | IMPORTANT |
| Gestational age at birth, weeks (Better indicated by higher values) | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none | 55 | 57 | - | MD 0.63 higher (0.41 to 0.85 higher) | MODERATE | IMPORTANT |
| Mode of birth (caesarean section) | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
17/55 (30.9%) |
21/57 (36.8%) | RR 0.84 (0.50 to 1.41) | 59 fewer per 1000 (from 184 fewer to 151 more) | VERY LOW | IMPORTANT |
| Superimposed pre-eclampsia | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | serious4 | none |
8/55 (14.5%) |
15/57 (26.3%) | RR 0.55 (0.25 to 1.20) | 118 fewer per 1000 (from 197 fewer to 53 more) | LOW | IMPORTANT |
| Superimposed pre-eclampsia < 34 weeks | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
6/55 (10.9%) |
6/57 (10.5%) | RR 1.04 (0.36 to 3.02) | 4 more per 1000 (from 67 fewer to 213 more) | VERY LOW | IMPORTANT |
| Eclampsia | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none |
0/55 (0%) |
0/57 (0%) | not calculable | not calculable | MODERATE | IMPORTANT |
| Maternal death | ||||||||||||
| 1 (Webster 2017) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none |
0/55 (0%) |
0/57 (0%) | not calculable | not calculable | MODERATE | IMPORTANT |
- 1
The quality of the evidence was downgraded by 1 level due to unclear risk of allocation concealment and a high risk of performance and detection bias
- 2
The quality of the evidence was downgraded by 2 levels as the 95% CI crossed 2 default MID thresholds (0.8 and 1.25)
- 3
The quality of the evidence was downgraded by 1 level as the 95% CI crossed 1 default MID threshold (883 × +/− 0.5= +/− 441.5)
- 4
95% CI crossed 1 default MID threshold (0.8)
Table 11Clinical evidence profile. Comparison 7. Labetalol versus methyldopa
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Labetalol | Methyldopa | Relative (95% CI) | Absolute | ||
| Stillbirth | ||||||||||||
| 1 (Moore 1982) | randomised trials | very serious1 | no serious inconsistency | serious2 | no serious imprecision | none |
0/38 (0%) |
0/34 (0%) | not calculable | not calculable | VERY LOW | CRITICAL |
| Neonatal death | ||||||||||||
| 1 (Moore 1982) | randomised trials | very serious1 | no serious inconsistency | serious2 | very serious3 | none |
2/38 (5.3%) |
0/34 (0%) | RR 4.49 (0.22 to 90.30)6 | - | VERY LOW | CRITICAL |
| Small for gestational age | ||||||||||||
| 2 (Moore 1982, Sibai 1990) | randomised trials | very serious1 | no serious inconsistency | serious2 | very serious3 | none |
20/124 (16.1%) |
21/122 (17.2%) | RR 0.89 (0.53 to 1.49) | 19 fewer per 1000 (from 81 fewer to 84 more) | VERY LOW | CRITICAL |
| Birth weight (grams) (Better indicated by higher values) | ||||||||||||
| 1 (Moore 1982) | randomised trials | very serious1 | no serious inconsistency | serious2 | no serious imprecision | none | 38 | 34 | - | MD 7 higher (363.32 lower to 377.32 higher) | VERY LOW | IMPORTANT |
| Gestational age at birth (weeks) (Better indicated by higher values) | ||||||||||||
| 1 (Moore 1982) | randomised trials | very serious1 | no serious inconsistency | serious2 | no serious imprecision | none | 38 | 34 | - | MD 0.1 higher (1.2 lower to 1.4 higher) | VERY LOW | IMPORTANT |
| Admission to neonatal unit | ||||||||||||
| 1 (Moore 1982) | randomised trials | very serious1 | no serious inconsistency | serious2 | very serious3 | none |
19/38 (50%) |
16/34 (47.1%) | RR 1.06 (0.66 to 1.71) | 28 more per 1000 (from 160 fewer to 334 more) | VERY LOW | IMPORTANT |
| Maximum sBP after entry (mmHg) (Better indicated by lower values) | ||||||||||||
| 1 (Moore 1982) | randomised trials | very serious1 | no serious inconsistency | serious2 | serious4 | none | 38 | 34 | - | MD 2.7 higher (5.82 lower to 11.22 higher) | VERY LOW | CRITICAL |
| Maximum dBP after entry (mmHg) (Better indicated by lower values) | ||||||||||||
| 1 (Moore 1982) | randomised trials | very serious1 | no serious inconsistency | serious2 | serious5 | none | 38 | 34 | - | MD 0.9 lower (5.99 lower to 4.19 higher) | VERY LOW | CRITICAL |
| Onset of labour (induction) | ||||||||||||
| 1 (Moore 1982) | randomised trials | very serious1 | no serious inconsistency | serious2 | very serious3 | none |
20/38 (52.6%) |
14/34 (41.2%) | RR 1.28 (0.77 to 2.11) | 115 more per 1000 (from 95 fewer to 457 more) | VERY LOW | IMPORTANT |
| Mode of birth (C-section) | ||||||||||||
| 2 (Moore 1982, Sibai 1990) | randomised trials | very serious1 | no serious inconsistency | serious2 | very serious3 | none |
49/124 (39.5%) |
51/122 (41.8%) | RR 0.93 (0.69 to 1.26) | 29 fewer per 1000 (from 130 fewer to 109 more) | VERY LOW | IMPORTANT |
- 1
The quality of the evidence was downgraded by 2 levels due to an unclear risk of random sequence generation, allocation concealment, performance and selection bias, and selective reporting
- 2
The quality of the evidence was downgraded by 1 level as 34.8% of participants did not present with chronic hypertension
- 3
The quality of the evidence was downgraded by 2 levels as the 95% CI crossed 2 default MID thresholds (0.8 and 1.25)
- 4
The quality of the evidence was downgraded by 1 level as the 95% CI crossed 1 MID threshold (14.9 × +/− 0.5 = +/− 7.45)
- 5
The quality of the evidence was downgraded by 1 level as the 95% CI crossed 1 default MID threshold (9.1 × +/− 0.5 = +/−4.55)
- 6
The corresponding absolute risk was not calculated as there were no events reported in the control arm.
Table 12Clinical evidence profile. Comparison 8. Methyldopa versus placebo
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Methyldopa | Placebo | Relative (95% CI) | Absolute | ||
| Stillbirth | ||||||||||||
| 1 (Weitz 1987) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none |
0/13 (0%) |
0/12 (0%) | not calculable | not calculable | MODERATE | CRITICAL |
| Neonatal death | ||||||||||||
| 1 (Weitz 1987) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none |
0/13 (0%) |
0/12 (0%) | not calculable | not calculable | MODERATE | CRITICAL |
| Gestational age at birth, weeks (Better indicated by higher values) | ||||||||||||
| 1 (Weitz 1987) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none | 13 | 12 | - | MD 1.43 higher (1.07 to 1.79 higher) | MODERATE | IMPORTANT |
| Superimposed pre-eclampsia | ||||||||||||
| 1 (Weitz 1987) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
5/13 (38.5%) |
4/12 (33.3%) | RR 1.15 (0.40 to 3.31) | 50 more per 1000 (from 200 fewer to 770 more) | VERY LOW | IMPORTANT |
- 1
The quality of the evidence was downgraded by 1 level due to an unclear risk of random sequence generation, allocation concealment and selective reporting
- 2
The quality of the evidence was downgraded by 2 levels as the 95% CI crossed 2 default MID thresholds (0.8 and 1.25)
Table 13Clinical evidence profile. Comparison 9. Methyldopa versus no intervention
| Quality assessment | No of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Methyldopa | No intervention | Relative (95% CI) | Absolute | ||
| Stillbirth | ||||||||||||
| 1 (Redman 1976) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | serious2 | none |
1/98 (1%) |
9/92 (9.8%) | RR 0.1 (0.01 to 0.81) | 88 fewer per 1000 (from 19 fewer to 97 fewer) | VERY LOW | CRITICAL |
| Perinatal death | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious3 | no serious inconsistency | no serious indirectness | very serious4 | none |
1/88 (1.1%) |
1/90 (1.1%) | RR 1.02 (0.06 to 16.10) | 0 more per 1000 (from 10 fewer to 168 more) | VERY LOW | CRITICAL |
| Small for gestational age | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious3 | no serious inconsistency | no serious indirectness | very serious4 | none |
6/88 (6.8%) |
8/90 (8.9%) | RR 0.77 (0.28 to 2.12) | 20 fewer per 1000 (from 64 fewer to 100 more) | VERY LOW | CRITICAL |
| Birth weight (grams) (Better indicated by higher values) | ||||||||||||
| 1 (Redman 1976) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none | 98 | 92 | - | MD 40 higher (117.58 lower to 197.58 higher) | LOW | IMPORTANT |
| Gestational age at birth (weeks) (Better indicated by higher values) | ||||||||||||
| 1 (Redman 1976) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none | 103 | 101 | - | MD 0.03 lower (0.48 lower to 0.42 higher) | LOW | IMPORTANT |
| Preterm birth (<37 weeks) | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious3 | no serious inconsistency | no serious indirectness | very serious4 | none |
11/88 (12.5%) |
9/90 (10%) | RR 1.25 (0.54 to 2.87) | 25 more per 1000 (from 46 fewer to 187 more) | VERY LOW | IMPORTANT |
| Impaired vision at 7.5 years old | ||||||||||||
| 1 (Cockburn 1982) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | serious2 | none |
7/98 (7.1%) |
14/92 (15.2%) | RR 0.47 (0.20 to 1.11) | 81 fewer per 1000 (from 122 fewer to 17 more) | VERY LOW | IMPORTANT |
| Impaired hearing at 7.5 years old | ||||||||||||
| 1 (Cockburn 1982) | randomised trials | very serious1 | no serious inconsistency | no serious indirectness | very serious4 | none |
7/96 (7.3%) |
6/92 (6.5%) | RR 1.12 (0.39 to 3.20) | 8 more per 1000 (from 40 fewer to 143 more) | VERY LOW | IMPORTANT |
| Superimposed pre-eclampsia | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious3 | no serious inconsistency | no serious indirectness | very serious4 | none |
16/88 (18.2%) |
14/90 (15.6%) | RR 1.17 (0.61 to 2.25) | 26 more per 1000 (from 61 fewer to 194 more) | VERY LOW | IMPORTANT |
| Placental abruption | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious3 | no serious inconsistency | no serious indirectness | very serious4 | none |
1/88 (1.1%) |
2/90 (2.2%) | RR 0.51 (0.05 to 5.54) | 11 fewer per 1000 (from 21 fewer to 101 more) | VERY LOW | IMPORTANT |
| Mode of birth (caesarean section) | ||||||||||||
| 1 (Sibai 1990) | randomised trials | very serious3 | no serious inconsistency | no serious indirectness | very serious4 | none |
31/88 (35.2%) |
29/90 (32.2%) | RR 1.09 (0.72 to 1.65) | 29 more per 1000 (from 90 fewer to 209 more) | VERY LOW | IMPORTANT |
- 1
The quality of the evidence was downgraded by 2 levels due to an unclear risk of random sequence generation, allocation concealment, performance and detection bias, and a high risk of selective reporting
- 2
The quality of the evidence was downgraded by 1 level as the 95% CI crossed 1 default MID threshold (0.8)
- 3
The quality of the evidence was downgraded by 2 levels due to an unclear risk of allocation concealment, performance and selection bias, and selective reporting
- 4
The quality of the evidence was downgraded by 2 levels as the 95% CI crossed 2 default MID thresholds (0.8 and 1.25)
Table 14Clinical evidence profile. Comparison 10. Amlodipine versus aspirin
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Amlodipine | Aspirin | Relative (95% CI) | Absolute | ||
| Stillbirth | ||||||||||||
| 1 (Vigil de Gracia 2014) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
0/20 (0%) |
1/19 (5.3%) | RR 0.32 (0.01 to 7.35) | 36 fewer per 1000 (from 52 fewer to 334 more) | VERY LOW | CRITICAL |
| Neonatal death | ||||||||||||
| 1 (Vigil de Gracia 2014) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | no serious imprecision | none |
0/20 (0%) |
0/19 (0%) | not calculable | not calculable | MODERATE | CRITICAL |
| Small-for-gestational age | ||||||||||||
| 1 (Vigil de Gracia 2014) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
2/20 (10%) |
2/19 (10.5%) | RR 0.95 (0.15 to 6.08) | 5 fewer per 1000 (from 89 fewer to 535 more) | VERY LOW | CRITICAL |
| Birth weight (grams) (Better indicated by higher values) | ||||||||||||
| 1 (Vigil de Gracia 2014) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | serious3 | none | 20 | 19 | - | MD 63 lower (467.79 lower to 341.79 higher) | LOW | IMPORTANT |
| Preterm birth (weeks not specified) | ||||||||||||
| 1 (Vigil de Gracia 2014) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
3/20 (15%) |
1/19 (5.3%) | RR 2.85 (0.32 to 25.07) | 97 more per 1000 (from 36 fewer to 1000 more) | VERY LOW | IMPORTANT |
| Severe hypertension | ||||||||||||
| 1 (Vigil de Gracia 2014) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
7/20 (35%) |
6/19 (31.6%) | RR 1.11 (0.45 to 2.70) | 35 more per 1000 (from 174 fewer to 537 more) | VERY LOW | CRITICAL |
| Placental abruption | ||||||||||||
| 1 (Vigil de Gracia 2014) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
1/20 (5%) |
0/19 (0%) | RR 2.86 (0.12 to 66.11)4 | - | VERY LOW | IMPORTANT |
| Mode of birth (caesarean section) | ||||||||||||
| 1 (Vigil de Gracia 2014) | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none |
12/20 (60%) |
10/19 (52.6%) | RR 1.14 (0.65 to 1.99) | 74 more per 1000 (from 184 fewer to 521 more) | VERY LOW | IMPORTANT |
- 1
The quality of the evidence was downgraded by 1 level due to a high risk of performance and selection bias and an unclear risk of selective reporting
- 2
The quality of the evidence was downgraded by 2 levels as the 95% CI crossed 2 default MID thresholds (0.8 and 1.25)
- 3
The quality of the evidence was downgraded by 1 level as the 95% CI crossed 1 default MID threshold (740 × +/− 0.5= +/− 370)
- 4
The corresponding absolute risk was not calculated as there were no events reported in the control arm.
Table 15Comparison 11. Aspirin versus no intervention
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Aspirin | Control | Relative (95% CI) | Absolute | ||
| Stillbirth and neonatal death | ||||||||||||
| 2 (Atallah 1996, Viinikka 1993) | randomised trials | no serious risk of bias | no serious inconsistency | serious1 | very serious2 | none |
24/330 (7.3%) |
17/326 (5.2%) | RR 1.36 (0.76 to 2.46) | 19 more per 1000 (from 13 fewer to 76 more) | VERY LOW | CRITICAL |
| Small-for-gestational age | ||||||||||||
| 4 (Atallah 1996, Moore 2015, Parazzini 1993, Viinikka 1993) | randomised trials | serious3 | no serious inconsistency | serious4 | serious5 | none |
63/557 (11.3%) |
68/517 (13.2%) | RR 0.82 (0.60 to 1.13) | 24 fewer per 1000 (from 53 fewer to 17 more) | VERY LOW | CRITICAL |
| Birthweight (grams; Better indicated by higher values) | ||||||||||||
| 1 (Viinikka 1993) | randomised trials | no serious risk of bias | no serious inconsistency | serious1 | serious6 | none | 97 | 100 | - | MD 178 higher (13.79 lower to 369.79 higher) | LOW | IMPORTANT |
| Gestational age (number of weeks; Better indicated by higher values) | ||||||||||||
| 1 (Viinikka 1993) | randomised trials | no serious risk of bias | no serious inconsistency | serious1 | no serious imprecision | none | 97 | 100 | - | MD 0.4 higher (0.17 lower to 0.97 higher) | MODERATE | IMPORTANT |
| Preterm birth <37 weeks | ||||||||||||
| 2 (Atallah 1996, Poon 2017) | randomised trials | no serious risk of bias | no serious inconsistency | serious7 | serious5 | none |
61/280 (21.8%) |
75/286 (26.2%) | RR 0.81 (0.60 to 1.08) | 50 fewer per 1000 (from 105 fewer to 21 more) | LOW | IMPORTANT |
| 1 (van Vliet 2017) | IPD meta-analysis of randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness | serious5 | none |
71/1266 (5.6%) |
94/1252 (7.5%) | RR 0.73 (0.53 to 1.00) | 20 fewer per 1000 (from 35 fewer to 0 more) | MODERATE | IMPORTANT |
| Preterm birth <34 weeks | ||||||||||||
| 1 (van Vliet 2017) | IPD meta-analysis of randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness | very serious2 | none |
21/1266 (1.7%) |
27/1252 (2.2%) | RR 0.77 (0.44 to 1.35) | 5 fewer per 1000 (from 12 fewer to 8 more) | LOW | IMPORTANT |
| Preterm birth <28 weeks | ||||||||||||
| 1 (van Vliet 2017) | IPD meta-analysis of randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness | very serious2 | none |
5/1266 (0.39%) |
9/1252 (0.72%) | RR 0.55 (0.18 to 1.63) | 3 fewer per 1000 (from 6 fewer to 5 more) | LOW | IMPORTANT |
| Admission to neonatal unit | ||||||||||||
| 1 (Viinikka 1993) | randomised trials | no serious risk of bias | no serious inconsistency | serious1 | serious5 | none |
10/97 (10.3%) |
21/100 (21%) | RR 0.49 (0.24 to 0.99) | 107 fewer per 1000 (from 2 fewer to 160 fewer) | LOW | IMPORTANT |
| Worsening of hypertension | ||||||||||||
| 1 (Viinikka 1993) | randomised trials | no serious risk of bias | no serious inconsistency | serious1 | very serious2 | none |
21/97 (21.6%) |
25/100 (25%) | RR 0.87 (0.52 to 1.44) | 32 fewer per 1000 (from 120 fewer to 110 more) | VERY LOW | CRITICAL |
| Diastolic BP at 36 weeks’ gestation (mmHg; Better indicated by lower values) | ||||||||||||
| 1 (Viinikka 1993) | randomised trials | no serious risk of bias | no serious inconsistency | serious1 | no serious imprecision | none | 97 | 100 | - | MD 0.2 lower (3.48 lower to 3.08 higher) | MODERATE | CRITICAL |
| Development of pre-eclampsia | ||||||||||||
| 2 (Poon 2017, Viinikka 1993) | randomised trials | no serious risk of bias | no serious inconsistency | serious8 | very serious2 | none |
14/146 (9.6%) |
16/161 (9.9%) | 0.96 (0.49 to 1.89) | 50 fewer per 1000 (from 105 fewer to 21 more) | VERY LOW | IMPORTANT |
| 1 (Askie 2007) | IPD meta-analysis of randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | none |
293/1678 (17.5%) |
295/1625 (18.2%) | 0.97 (0.84 to 1.12) | 5 fewer per 1000 (from 29 fewer to 22 more) | HIGH | IMPORTANT |
| Spontaneous onset of labour | ||||||||||||
| 1 (Viinikka 1993) | randomised trials | no serious risk of bias | no serious inconsistency | serious1 | serious9 | none |
45/97 (46.4%) |
40/100 (40%) | RR 1.16 (0.84 to 1.60) | 64 more per 1000 (from 64 fewer to 240 more) | LOW | IMPORTANT |
- 1
The quality of the evidence was downgraded by 1 level as the study by Viinikka et al. 1993 included a mixed population of women, 89% of whom had chronic hypertension, and 11% had a history of pre-eclampsia in a previous pregnancy.
- 2
The quality of the evidence was downgraded by 2 levels as the 95% CI crosses 2 default MID thresholds (0.8 and 1.25)
- 3
The quality of the evidence was downgraded by 1 level as one study was at high risk of performance and detection bias (open label study)
- 4
Note that the outcomes reported have slight differences in the individual trials: Moore 2015 and Parazzini 1993 report <10th centile, Atallah 1996 reports <3rd centile and Viinikka 1993 reports <2SD below the mean for gestational age.
- 5
The quality of the evidence was downgraded by 1 level as the 95% CI crosses 1 default MID threshold (0.8)
- 6
The quality of the evidence was downgraded by 1 level as the 95% CI crosses 1 MID threshold (MID calculated as 0.5 × 665 = +/−332.5g)
- 7
Note that the outcomes reported have slight differences for individual trials: Atallah 1996 reports on all preterm delivery <37 weeks, Poon 2017 reports on preterm birth <37 weeks due to pre-eclampsia
- 8
Note that the outcomes reported have slight differences in the individual trials: Askie 2007 reports on hypertension with new onset proteinuria after 20 weeks’ gestation, Poon 2017 reports on delivery with pre-eclampsia before 37 weeks’ gestation.
- 9
The quality of the evidence was downgraded by 1 level as the 95% CI crosses 1 default MID threshold (1.25)
Appendix H. Economic evidence tables
No economic evidence was identified for this review question.
Appendix I. Health economic evidence profiles
No economic evidence was identified for this review question.
Appendix J. Health economic analysis
No health economic analysis was conducted for this review question.
Appendix K. Excluded studies
Clinical studies
Table 16Clinical excluded studies with reasons for exclusion
| Study | Reason for exclusion |
|---|---|
| Aalami-Harandi, Rezvan, Karamali, Maryam, Asemi, Zatollah, The favorable effects of garlic intake on metabolic profiles, hs-CRP, biomarkers of oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia: randomized, double-blind, placebo-controlled trial, The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians, 28, 2020-7, 2015 | Women with chronic hypertension were not included |
| Abalos,E., Duley,L., Steyn,D.W., Henderson-Smart,D.J., Antihypertensive drug therapy for mild to moderate hypertension during pregnancy, Cochrane Database of Systematic Reviews, 2007. Article Number, -, 2007 | Studies included covered women with any type of hypertensive disorder. Studies including women with chronic hypertension have been considered for inclusion in this systematic review |
| Abramovici, Adi, Jauk, Victoria, Wetta, Luisa, Cantu, Jessica, Edwards, Rodney, Biggio, Joseph, Tita, Alan, Low-dose aspirin, smoking status, and the risk of spontaneous preterm birth, American Journal of Perinatology, 32, 445-50, 2015 | No stratified analysis for women with chronic hypertension. Compares smokers and non-smokers only. |
| Allshouse, A. A., Jessel, R. H., Heyborne, K. D., The impact of low-dose aspirin on preterm birth: Secondary analysis of a randomized controlled trial, Journal of Perinatology, 36, 427-31, 2016 | Only 41.5% of participants had chronic hypertension. No stratified analysis for women with chronic hypertension. |
| Anca-Daniela, S., Banica, R., Sima, R. M., Ples, L., Low dose aspirin for preventing fetal growth restriction: A randomised trial, Journal of Perinatal Medicine, 43, 2015 | Participants had high risk first trimester screening result. No data on prevalence of chronic hypertension in population, and no stratified analysis for women with chronic hypertension. |
| Anonymous,, Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Gruppo di Studio Ipertensione in Gravidanza, British Journal of Obstetrics and Gynaecology, 105, 718-22, 1998 | Less than 66% of participants presented with chronic hypertension |
| Anonymous,, CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group, Lancet (London, England), 343, 619-29, 1994 | 20% of participants had chronic hypertension. No stratified analysis for this group of women only |
| Anonymous,, Low dose aspirin in pregnancy and early childhood development: follow up of the collaborative low dose aspirin study in pregnancy. CLASP collaborative group, British Journal of Obstetrics and Gynaecology, 102, 861-8, 1995 | 20% of participants had chronic hypertension, but results are not presented with stratified analysis for this group of women. |
| Aparna, J., A randomized, double-blind, comparative trial of nifedipine and methyldopa in moderate pregnancy induced hypertension, Der Pharmacia Lettre, 5, 274-277, 2013 | Paper unavailable |
| Arias, F., Zamora, J., Antihypertensive treatment and pregnancy outcome in patients with mild chronic hypertension, Obstetrics and Gynecology, 53, 489-94, 1979 | Some of the participants received hydroclorothiazide |
| Atallah, A., Lecarpentier, E., Goffinet, F., Doret-Dion, M., Gaucherand, P., Tsatsaris, V., Aspirin for Prevention of Preeclampsia, Drugs, 77, 1819-1831, 2017 | Narrative review article. |
| Baker, P. A., Chadd, M. A., Humphreys, D. M., Leather, H. M., Controlled trial of hypotensive agents in hypertension in pregnancy, British heart journal, 30, 871, 1968 | Abstract |
| Baschat, A. A., Dewberry, D., Seravalli, V., Miller, J. L., Block-Abraham, D., Blitzer, M. G., Maternal blood pressure trends throughout pregnancy and development of pre-eclampsia in women receiving first trimester aspirin prophylaxis, Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology, 2017 | Only 14.8% of participants had chronic hypertension, and no stratified analysis is presented for this subgroup. |
| Beaufils, M., Donsimoni, R., Uzan, S., Colau, J. C., Prevention of pre-elcampsia by early antiplatelet therapy, Lancet, 1, 840-842, 1985 | Participants were recruited due to obstetric history (stillbirth, IUGR or miscarriage). Only 1 participant had hypertension. |
| Bergel, E., Carroli, G., Althabe, F., Ambulatory versus conventional methods for monitoring blood pressure during pregnancy, Cochrane Database of Systematic Reviews, CD001231, 2002 | No trials were included |
| Bijvank,S.W.A.N., Duvekot,J.J., Nicardipine for the treatment of severe hypertension in pregnancy: A review of the literature, Obstetrical and Gynecological Survey, 65, 341-347, 2010 | The studies included in this review were either not randomised or included women with pre-eclampsia |
| Bonnin, P, Mintz, P, Kedra, Aw, Pruna, A, Ciraru-Vigneron, N, Savin, E, Lefevre, V, Szyller, A, Belmont, C, Ferrand, S, Ravina, Jh, Idatte, Jm, Bailliart, O, Martineaud, Jp, Effects of nifedipine and atenolol on the fetal-maternal circulation in moderate hypertension in pregnancy, Therapie, 45, 525, 1990 | Paper unavailable |
| Bortolus, R., Ricci, E., Chatenoud, L., Parazzini, F., Nifedipine administered in pregnancy: Effect on the development of children at 18 months, British Journal of Obstetrics and Gynaecology, 107, 792-794, 2000 | Follow-up of a study that presented with less than 66% of participants presented with chronic hypertension |
| Brennecke, S. P., Brown, M. A., Crowther, C. A., Hague, W. M., King, J., McCowan, L., Morris, J., North, R., Pattison, N., Tippett, C., Wilson, D., Aspirin and prevention of preeclampsia, Australian and New Zealand Journal of Obstetrics and Gynaecology, 35, 38-41, 1995 | Position statement only, no analysis or clinical data reported. |
| Broekhuijsen, K., Van Baaren, G. J., Van Pampus, M., Sikkema, M., Woiski, M., Oudijk, M., Bloemenkamp, K., Scheepers, H., Bremer, H., Rijnders, R., Van Loon, A., Perquin, D., Sporken, J., Papatsonis, D., Van Huizen, M., Vredevoogd, C., Brons, J., Van Kaam, A., Groen, H., Porath, M., Mol, B., Franssen, M., Langenveld, J., Delivery versus expectant monitoring for late preterm hypertensive disorders of pregnancy (HYPITAT-II): A multicenter, open label, randomized controlled trial, American Journal of Obstetrics and Gynecology, 210, S2-S3, 2014 | Abstract |
| Brown, M. A., Budle, M. L., Cario, G. M., Whitworth, J. A., Ambulatory blood pressure monitoring during pregnancy. Comparison with mercury sphygmomanometry, American Journal of Hypertension, 6, 745-749, 1993 | Not a randomised trial |
| Brown, M. A., Roberts, L. M., Mackenzie, C., Mangos, G., Davis, G. K., A prospective randomized study of automated versus mercury blood pressure recordings in hypertensive pregnancy (PRAM Study), Hypertension in Pregnancy, 31, 107-19, 2012 | Less than 66% of participants presented with chronic hypertension |
| Brown,M.A., Buddle,M.L., Farrell,T., Davis,G.K., Efficacy and safety of nifedipine tablets for the acute treatment of severe hypertension in pregnancy, American Journal of Obstetrics and Gynecology, 187, 1046-1050, 2002 | Study compared two different types of nifedipine tables |
| Bujold, E., Roberge, S., Nicolaides, K. H., Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation, Prenatal Diagnosis, 34, 642-8, 2014 | Review article. No subgroup analysis for women with chronic hypertension. |
| Bujold,E., Roberge,S., Lacasse,Y., Bureau,M., Audibert,F., Marcoux,S., Forest,J.C., Giguere,Y., Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis, Obstetrics and Gynecology, 116, 402-414, 2010 | No subgroup analysis for women with chronic hypertension. |
| Byaruhanga, R. N., Chipato, T., Rusakaniko, S., A randomized controlled trial of low-dose aspirin in women at risk from pre-eclampsia, International Journal of Gynecology and Obstetrics, 60, 129-135, 1998 | Relevant data from this trial is included in the IPD meta-analysis by Askie et al. 2007. |
| Cameron, Ad, Walker, Jj, Bonduelle, M, Calder, Aa, A randomised trial of the antihypertensive agent, labetalol, against bed rest in pregnancy hypertension, Archives of gynecology, 237 Suppl, 295, 1985 | Abstract |
| Cantu, J. A., Jauk, V. R., Owen, J., Biggio, J. R., Abramovici, A. R., Edwards, R. K., Tita, A. T., Is low-dose aspirin therapy to prevent preeclampsia more efficacious in non-obese women or when initiated early in pregnancy?, Journal of Maternal-Fetal & Neonatal Medicine, 28, 1128-1132, 2015 | Relevant data are included in the IPD meta-analysis by Askie et al 2007 |
| Cao, N. T., Vu, Q. H. N., Truong, Q. V., Vo, V. D., Tran, M. L., Effectiveness of low-dose aspirin for the prevention of pre-eclampsia, Journal of Obstetrics and Gynaecology Research, 43, 69, 2017 | Conference abstract |
| Carbonne, B., Jannet, D., Touboul, C., Khelifati, Y., Milliez, J., Nicardipine treatment of hypertension during pregnancy, Obstetrics and Gynecology, 81, 908-14, 1993 | Not a randomised trial |
| Caritis, S., Sibai, B., Hauth, J., Lindheimer, M. D., Klebanoff, M., Thom, E., Vandorsten, P., Landon, M., Paul, R., Miodovnik, M., Meis, P., Thurnau, G., Bottoms, S., McNellis, D., Roberts, J. M., Low-dose aspirin to prevent preeclampsia in women at high risk, New England Journal of Medicine, 338, 701-705, 1998 | Relevant subgroup analysis from this trial in included in the papers by Askie et al 2007 (and van Vliet 2017). |
| Chiaffarino, F., Parazzini, F., Paladini, D., Acaia, B., Ossola, W., Marozio, L., Facchinetti, F., Giudice, A. D., A small randomised trial of low-dose aspirin in women at high risk of pre-eclampsia, European Journal of Obstetrics Gynecology and Reproductive Biology, 112, 142-144, 2004 | No stratified analysis for participants with chronic hypertension. |
| Ciraru-Vigneron, N, Pruna, A, Akposso, K, Bonnin, P, Kedra, W, Mintz, P, Ferrand, S, Smadja, S, Martineaud, Jp, Idatte, Jm, Ravina,, Comparison of the effects of nefedipine and atenolol in the treatment of uncomplicated hypertension in pregnancy, Therapie, 47, 221, 1992 | Paper unavailable |
| Cluver, C., Novikova, N., Koopmans, C. M., West, H. M., Planned early delivery versus expectant management for hypertensive disorders from 34 weeks gestation to term, Cochrane Database of Systematic Reviews, 2017, CD009273, 2017 | Mixed population of women with PE, GE and CHT. The study that included women with CHT has already been included in this systematic review (Hamed 2014) |
| Coomarasamy,A., Honest,H., Papaioannou,S., Gee,H., Khan,K.S., Aspirin for prevention of preeclampsia in women with historical risk factors: A systematic review, Obstetrics and Gynecology, 101, 1319-1332, 2003 | No subgroup analysis for women with chronic hypertension. |
| Cristina Rossi, A., D’Addario, V., Prevention of preeclampsia with low-dose aspirin or vitamins C/E: A systematic review with metaanalysis, American Journal of Obstetrics and Gynecology, 201, S266-S267, 2009 | No subgroup analysis for women with chronic hypertension. |
| Cruickshank, D. J., Campbell, D., Robertson, A. A., MacGillivray, I., Intra-uterine growth retardation and maternal labetalol treatment in a random allocation controlled study, Journal of Obstetrics and Gynaecology, 12, 223-227, 1992 | Women presented with gestational hypertension |
| Cruickshank, D. J., Robertson, A. A., Campbell, D. M., MacGillivray, I., Does labetalol influence the development of proteinuria in pregnancy hypertension? A randomised controlled study, European journal of obstetrics, gynecology, and reproductive biology, 45, 47-51, 1992 | Women presented with gestational hypertension |
| Cruickshank, Dj, Campbell, Dm, Atenolol in essential hypertension during pregnancy, BMJ (Clinical research ed.), 301, 1103, 1990 | Women presented with gestational hypertension |
| Cruickshank,D.J., Robertson,A.A., Campbell,D.M., MacGillivray,I., Maternal obstetric outcome measures in a randomised controlled study of labetalol in the treatment of hypertension in pregnancy, Clinical and Experimental Hypertension - Part B Hypertension in Pregnancy, 10, 333-344, 1991 | Women presented with gestational hypertension |
| da Silva, S. G., Hallal, P. C., Domingues, M. R., Bertoldi, A. D., Silveira, M. F., Bassani, D., da Silva, I. C. M., da Silva, B. G. C., Coll, C. V. N., Evenson, K., A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: Results from the PAMELA study, International Journal of Behavioral Nutrition and Physical Activity, 14, 175, 2017 | Women with CHT were not included |
| da Silva, Shana G., Ricardo, Luiza I., Evenson, Kelly R., Hallal, Pedro C., Leisure-Time Physical Activity in Pregnancy and Maternal-Child Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Cohort Studies, Sports medicine (Auckland, N.Z.), 47, 295-317, 2017 | Women with CHT were not included |
| Di Mascio, D., Magro-Malosso, E. R., Saccone, G., Marhefka, G. D., Berghella, V., Exercise during pregnancy in normal-weight women and risk of preterm birth: a systematic review and meta-analysis of randomized controlled trials, American Journal of Obstetrics and Gynecology, 215, 561-571, 2016 | Women with CHT were not included |
| Duggan,P.M., McCowan,L.M., Stewart,A.W., Antihypertensive drug effects on placental flow velocity waveforms in pregnant women with severe hypertension, Australian and New Zealand Journal of Obstetrics and Gynaecology, 32, 335-338, 1992 | Non extractable data (only p-values have been reported) |
| Duley, L., Henderson-Smart, D. J., Meher, S., King, J. F., Antiplatelet agents for preventing pre-eclampsia and its complications, Cochrane Database of Systematic Reviews, CD004659, 2007 | No data on number of women with chronic hypertension, and no subgroup analysis for this group of women. |
| Duley, L., Meher, S., Jones, L., Drugs for treatment of very high blood pressure during pregnancy, Cochrane Database of Systematic Reviews, CD001449, 2013 | The majority of studies included in this review are not relevant for the protocol either because are abstracts, have been published in a foreign language or have no relevant interventions. The remaining studies have been considered for inclusion in this systematic review |
| Ebrashy,A., Ibrahim,M., Marzook,A., Yousef,D., Usefulness of aspirin therapy in high-risk pregnant women with abnormal uterine artery Doppler ultrasound at 14-16 weeks pregnancy: randomized controlled clinical trial, Croatian Medical Journal, 46, 826-831, 2005 | 35% participants had chronic hypertension. No subgroup analysis is reported for these women. |
| El Guindy, A. A., Nabhan, A. F., A randomized trial of tight vs. less tight control of mild essential and gestational hypertension in pregnancy, Journal of Perinatal Medicine, 36, 413-418, 2008 | Less than 66% of participants presented with chronic hypertension |
| Elder, M. G., de Swiet, M., Sullivan, M., A randomised trial of low dose aspirin for primiparae in pregnancy (Golding)/Barbados low dose aspirin study in pregnancy (BLASP) (Rotchell et al.), British Journal of Obstetrics and Gynaecology, 106, 180, 1999 | Women did not present with CHT |
| Farrell, B., Heineman, J., Handoll, H., Pearson, M., Collingwood, M., Belcher, J., Grant, A., Mutch, L., De Swiet, M., Redman, C., Collins, R., Elder, M., Rubin, P., Symonds, M., Wallenberg, H., Doll, R., Chalmers, I., Elstein, M., Peto, R., Low dose aspirin in pregnancy and early childhood development: Follow up of the collaborative low dose aspirin study in pregnancy, British Journal of Obstetrics and Gynaecology, 102, 861-868, 1995 | <20% participants had chronic hypertension. No subgroup analysis reported for these women. |
| Finnstrom, O., Ezitis, J., Ryden, G., Wichman, K., Neonatal effects of beta-blocking drugs in pregnancy, Acta Obstetricia et Gynecologica Scandinavica - Supplement, 118, 91-3, 1984 | No relevant intervention (metoprolol) |
| Firoz, T., Magee, L. A., Lalani, S., Sawchuck, D., Payne, B., Vidler, M., Gordon, R., Von Dadelszen, P., Oral antihypertensive therapy for severe hypertension in pregnancy, Pregnancy Hypertension, 2, 288, 2012 | Some of the studies in this review are not relevant for the protocol either because included women with PE, published in a foreign language or presented with no relevant interventions. The relevant studies have been considered for inclusion |
| Fitton, C. A., Steiner, M. F. C., Aucott, L., Pell, J. P., Mackay, D. F., Fleming, M., McLay, J. S., In-utero exposure to antihypertensive medication and neonatal and child health outcomes: a systematic review, Journal of Hypertension, 11, 11, 2017 | The majority of studies in this review are not relevant for the protocol either because included women with PE or presented with no relevant interventions. The relevant trials have been considered for inclusion |
| Gallery,E.D.M., Ross,M.R., Hawkins,M., Leslie,G., Gyory,A.Z., Low-dose aspirin in high-risk pregnancy?, Hypertension in Pregnancy, 16, 229-238, 1997 | 55.5% participants had chronic hypertension, but no stratified analysis is presented for these women. |
| Golding, J., A randomised trial of low dose aspirin for primiparae in pregnancy, British Journal of Obstetrics and Gynaecology, 105, 293-299, 1998 | No data on number of participants with chronic hypertension, or subgroup analysis for these women. |
| Gonzalez, Jc, Andolcetti, R, Labetalol vs alpha methyldopa in the treatment of hypertension in pregnancy, Boletin medico de postgrado, 13, 3-8, 1997 | Study in Spanish |
| Grab, D., Paulus, W. E., Erdmann, M., Terinde, R., Oberhoffer, R., Lang, D., Muche, R., Kreienberg, R., Effects of low-dose aspirin on uterine and fetal blood flow during pregnancy: Results of a randomized, placebo-controlled, double-blind trial, Ultrasound in Obstetrics and Gynecology, 15, 19-27, 2000 | No data on number of women included with chronic hypertension. |
| Gresham, E., Bisquera, A., Byles, J. E., Hure, A. J., Effects of dietary interventions on pregnancy outcomes: a systematic review and meta-analysis, Maternal & Child Nutrition, 12, 5-23, 2016 | The studies included were not specific to women presenting with chronic hypertenion |
| Gresham, E., Bisquera, A., Hure, A., Byles, J., Gil, A., Martinez, J. A., A systematic review and meta-analysis of dietary intervention during pregnancy on maternal hypertensive disorders and preterm delivery, Annals of Nutrition and Metabolism, 63, 607, 2013 | Abstract |
| Haapsamo,M., Martikainen,H., Tinkanen,H., Heinonen,S., Nuojua-Huttunen,S., Rasanen,J., Low-dose aspirin therapy and hypertensive pregnancy complications in unselected IVF and ICSI patients: a randomized, placebo-controlled, double-blind study, Human Reproduction, 25, 2972-2977, 2010 | Women did not present with chronic hypertension |
| Henderson, J. T., Whitlock, E. P., O’Connor, E., Senger, C. A., Thompson, J. H., Rowland, M. G., Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the u.s. preventive services task force, Annals of Internal Medicine, 160, 695-703, 2014 | No subgroup analysis presented for women with chronic hypertension. |
| Hennessy,A., Thornton,C.E., Makris,A., Ogle,R.F., Henderson-Smart,D.J., Gillin,A.G., Child,A., A randomised comparison of hydralazine and mini-bolus diazoxide for hypertensive emergencies in pregnancy: the PIVOT trial, Australian and New Zealand Journal of Obstetrics and Gynaecology, 47, 279-285, 2007 | No relevant intervention (diazoxide) |
| Hermida, R. C., Ayala, D. E., Fernandez, J. R., Mojon, A., Alonso, I., Silva, I., Ucieda, R., Codesido, J., Iglesias, M., Administration time-dependent effects of aspirin in women at differing risk for preeclampsia, Hypertension, 34, 1016-23, 1999 | No data on muber of participants with chronic hypertension. No subgroup analysis for women with chronic hypertension. |
| Holbrook, B., Nirgudkar, P., Mozurkewich, E., Efficacy of hydralazine, labetalol, and nifedipine for the acute reduction of severe hypertension in pregnancy: A systematic review, American Journal of Obstetrics and Gynecology, 212, S287, 2015 | Abstract |
| Horvath, J. S., Phippard, A., Korda, A., Clonidine hydrochloride - A safe and effective antihypertensive agent in pregnancy, Obstetrics and Gynecology, 66, 634-638, 1985 | No relevant intervention (clonidine) |
| Imperiale,T.F., Petrulis,A.S., A meta-analysis of low-dose aspirin for the prevention of pregnancy-induced hypertensive disease, JAMA, 266, 260-264, 1991 | No subgroup analysis for women with chronic hypertension |
| Jabeen, M., Yakoob, M. Y., Imdad, A., Bhutta, Z. A., Impact of interventions to prevent and manage preeclampsia and eclampsia on stillbirths, BMC Public Health, 11 Suppl 3, S6, 2011 | No subgroup analysis for women with chronic hypertension. |
| Jiang, N., Liu, Q., Liu, L., Yang, W. W., Effect of calcium channel blockers plus low-dosage aspirin on hypertensive pregnancy outcomes, Obstetrics and Gynecology, 123, 57S, 2014 | Abstract |
| Kasawara, K. T., Burgos, C. S. G., Nascimento, S. L., Costa, M. L., Surita, F., E Silva, J. L. Pinto, OS020. Effects of exercise on maternal and neonatal outcomes in pregnantwomen with chronic hypertension and/or previous preecampsia: A randomized clinical trial, Pregnancy Hypertension, 2, 185-6, 2012 | Abstract |
| Koren,G., Systematic review of the effects of maternal hypertension in pregnancy and antihypertensive therapies on child neurocognitive development, Reproductive Toxicology, 39, 1-5, 2013 | This review included not relevant studies, asesseing the effects of maternal hypertension in pregnancy. For those studies assessing the relationship between antihypertensive medications and neurodevelopmental outcomes, not all of them were relevant for the study protocol. Those which are relevant have been asessed for inclusion |
| Leather, H. M., Humphreys, D. M., Baker, P., Chadd, M. A., A controlled trial of hypotensive agents in hypertension in pregnancy, Lancet, 2, 488-90, 1968 | For most of the relevant outcomes, data was not presented stratified by CHT, SDs were not reported for continuous outcomes. |
| Leslie, G. I., Gallery, E. D., Arnold, J. D., Ross, M. R., Gyory, A. Z., Neonatal outcome in a randomized, controlled trial of low-dose aspirin in high-risk pregnancies, Journal of Paediatrics & Child Health, 31, 549-52, 1995 | No data on number of participants with chronic hypertension. |
| Liu, F. M., Zhao, M., Wang, M., Yang, H. L., Li, L., Effect of regular oral intake of aspirin during pregnancy on pregnancy outcome of high-risk pregnancy-induced hypertension syndrome patients, European Review for Medical & Pharmacological Sciences, 20, 5013-5016, 2016 | Women with chronic (pre-existing) hypertension were excluded. |
| Liu, F., Yang, H., Li, G., Zou, K., Chen, Y., Effect of a small dose of aspirin on quantitative test of 24-h urinary protein in patients with hypertension in pregnancy, Experimental and Therapeutic Medicine, 13, 37-40, 2017 | No data on number of participants with chronic hypertension. |
| Liu, J., Trivedi, T., Blair, S. N., Ness, A., Macdonald-Wallis, C., Lawlor, D. A., Physical activity and hypertensive disorders of pregnancy among british women, American Journal of Epidemiology, 175, S22, 2012 | Abstract |
| Luchini, L., Bortolus, R., Parazzini, F., Multicentric, randomized, clinical trial on the efficacy of long-acting nifedipine in improving the prognosis of pregnancy in women with mild or moderate, chronic or pregnancy-induced hypertension, Journal of Nephrology, 6, 51-54, 1993 | Study proposal |
| Magee,L.A., Duley,L., Oral beta-blockers for mild to moderate hypertension during pregnancy, Cochrane database of systematic reviews (Online), 2003. Date of Publication, -, 2003 | The majority of studies included in this review are not relevant for the protocol either because are abstracts, have been published in a foreign language or have no relevant interventions. The remaining studies have been considered for inclusion in this systematic review |
| Magee,L.A., Elran,E., Bull,S.B., Logan,A., Koren,G., Risks and benefits of beta-receptor blockers for pregnancy hypertension: Overview of the randomized trials, European Journal of Obstetrics Gynecology and Reproductive Biology, 88, 15-26, 2000 | The majority of studies included in this review are not relevant for the protocol either because are abstracts, have been published in a foreign language or have no relevant interventions. The remaining studies have been considered for inclusion in this systematic review |
| Meher, S., Duley, L., Hunter, K., Askie, L., Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: an individual participant data meta-analysis, American Journal of Obstetrics & Gynecology, 216, 121-128.e2, 2017 | No subgroup data for women with chronic hypertension. |
| Mutch, L. M., Moar, V. A., Ounsted, M. K., Redman, C. W., Hypertension during pregnancy, with and without specific hypotensive treatment. II. The growth and development of the infant in the first year of life, Early human development, 1, 59-67, 1977 | Most of the participants included in this trial overlapped with those included in the Redman 1976 trial |
| Mutch,L.M., Moar,V.A., Ounsted,M.K., Redman,C.W., Hypertension during pregnancy, with and without specific hypotensive treatment. I. Perinatal factors and neonatal morbidity, Early Human Development, 1, 47-57, 1977 | Most of the participants included in this trial overlapped with those included in the Redman 1976 trial |
| Nielsen, L. H., Ovesen, P., Hansen, M. R., Brantlov, S., Jespersen, B., Bie, P., Jensen, B. L., Changes in the renin-angiotensin-aldosterone system in response to dietary salt intake in normal and hypertensive pregnancy. A randomized trial, Journal of the american society of hypertension, 10, 881-890.e4, 2016 | Women with chronic hypertension were not included |
| Nij, Bijvank Sw, Duvekot, Jj, Nicardipine for the treatment of severe hypertension in pregnancy: a review of the literature (Provisional abstract), Obstetrical and Gynecological Survey, 65, 341-347, 2010 | This review included observational studies only |
| Novikova, N., Cluver, C., Koopmans, C. M., Delivery versus expectant management for hypertensive disorders from 34 weeks gestation to term, Cochrane Database of Systematic Reviews, 2011, CD009273, 2011 | The majority of studies included in this review are not relevant for the protocol either because are abstracts, have been published in a foreign language or have no relevant interventions. The remaining studies have been considered for inclusion in this systematic review |
| Odibo, A. O., Goetzinger, K. R., Odibo, L., Tuuli, M. G., Early prediction and aspirin for prevention of pre-eclampsia (EPAPP) study: a randomized controlled trial, Ultrasound in Obstetrics & Gynecology, 46, 414-8, 2015 | 53% of participants had chronic hypertension. No subgroup analysis presented for these women. |
| Park, F., Russo, K., Pellosi, M., Puddephat, R., Walter, M., Leung, C., Saiid, R., Rawashdeh, H., Hyett, J., The impact of aspirin on the prevalence of early onset pre-eclampsia after first trimester screening, Prenatal Diagnosis, 34, e4, 2014 | No subgroup analysis for women with chronic hypertension. |
| Patel, P., Koli, D., Maitra, N., Sheth, T., Vaishnav, P., Comparison of Efficacy and Safety of Intravenous Labetalol Versus Hydralazine for Management of Severe Hypertension in Pregnancy, Journal of Obstetrics and Gynecology of India, 1-6, 2017 | No relevant comparator (hydralazine) |
| Peacock, Iv W. F., Hilleman, D. E., Levy, P. D., Rhoney, D. H., Varon, J., A systematic review of nicardipine vs labetalol for the management of hypertensive crises, American Journal of Emergency Medicine, 30, 981-993, 2012 | This study did not cover women with chronic hypertension |
| Phippard, A. F., Fischer, W. E., Horvath, J. S., Child, A. G., Korda, A. R., Henderson-Smart, D., Duggin, G. D., Tiller, D. J., Early blood pressure control improves pregnancy outcome in primigravid women with mild hypertension, Medical Journal of Australia, 154, 378-382, 1991 | Women presented with gestational hypertension |
| Pickles, C. J., Broughton Pipkin, F., Symonds, E. M., A randomised placebo controlled trial of labetalol in the treatment of mild to moderate pregnancy induced hypertension, British Journal of Obstetrics & Gynaecology, 99, 964-8, 1992 | Women presented with gestational hypertension |
| Raheem, I. A., Saaid, R., Omar, S. Z., Tan, P. C., Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomised trial, BJOG: An International Journal of Obstetrics & Gynaecology, 119, 78-85, 2012 | Women presented with gestational hypertension |
| Ramaiya, C., Mgaya, H. N., Low dose aspirin in prevention of pregnancy-induced hypertension in primigravidae at the Muhimbili Medical Center, Dar es Salaam, East African medical journal, 72, 690-3, 1995 | No data on number of participants with chronic hypertension. |
| Redman, C. W., Beilin, L. J., Bonnar, J., Treatment of hypertension in pregnancy with methyldopa: blood pressure control and side effects, British Journal of Obstetrics & Gynaecology, 84, 419-26, 1977 | No relevant outcomes were reported |
| Rey,E., Morin,F., Boudreault,J., Pilon,F., Vincent,D., Ouellet,D., Blood pressure assessments in different subtypes of hypertensive pregnant women: office versus home patient- or nurse-measured blood pressure, Hypertension in Pregnancy, 28, 168-177, 2009 | Observational study |
| Rezaei, Z., Sharbaf, F. R., Pourmojieb, M., Youefzadeh-Fard, Y., Motevalian, M., Khazaeipour, Z., Esmaeili, S., Comparison of the efficacy of nifedipine and hydralazine in hypertensive crisis in pregnancy, Acta Medica Iranica, 49, 701-6, 2011 | No relevant comparison (hydralazine) |
| Rhodes, C. A., Beevers, D. G., Churchill, D., A randomized trial of ambulatory blood pressure monitoring versus clinical blood pressure measurement in the management of hypertension in pregnancy. A feasibility study, Pregnancy Hypertension, 2017 | Less than 66% of participants presented with chronic hypertension |
| Roberge, S., Bujold, E., Nicolaides, K. H., Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis, American Journal of Obstetrics and Gynecology, 2017 | No subgroup analysis for women with chronic hypertension. |
| Roberge, S., Giguere, Y., Villa, P., Nicolaides, K., Vainio, M., Forest, J. C., Von Dadelzen, P., Vaiman, D., Tapp, S., Bujold, E., Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: A systematic review and meta-analysis, American Journal of Perinatology, 29, 551-556, 2012 | No information on number of women with chronic hypertension, or subgroup analysis for these women. |
| Roberge, S., Nicolaides, K. H., Demers, S., Villa, P., Bujold, E., Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis, Ultrasound in Obstetrics & Gynecology, 41, 491-9, 2013 | No subgroup analysis for women with chronic hypertension. |
| Roberge, S., Nicolaides, K., Demers, S., Hyett, J., Chaillet, N., Bujold, E., The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis, American Journal of Obstetrics & Gynecology, 216, 110-120.e6, 2017 | No subgroup analysis for women with chronic hypertension. |
| Roberge, S., Sibai, B., McCaw-Binns, A., Bujold, E., Low-Dose Aspirin in Early Gestation for Prevention of Preeclampsia and Small-for-Gestational-Age Neonates: Meta-analysis of Large Randomized Trials, American Journal of Perinatology, 33, 781-785, 2016 | No subgroup analysis for women with chronic hypertension. |
| Roberge, S., Villa, P., Nicolaides, K., Giguere, Y., Vainio, M., Bakthi, A., Ebrashy, A., Bujold, E., Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis, Fetal Diagnosis & Therapy, 31, 141-6, 2012 | No subgroup analysis for women with chronic hypertension. |
| Roberge, Stephanie, Bujold, Emmanuel, Nicolaides, Kypros H., Meta-analysis on the effect of aspirin use for prevention of preeclampsia on placental abruption and antepartum hemorrhage, American Journal of Obstetrics and Gynecology, 2018 | No subgroup analysis for women with chronic hypertension. |
| Rogers, M. S., Fung, H. Y., Hung, C. Y., Calcium and low-dose aspirin prophylaxis in women at high risk of pregnancy-induced hypertension, Hypertension in Pregnancy, 18, 165-72, 1999 | Women with chronic hypertension were not included. |
| Rolnik, Dl, Wright, D, Poon, Lc, O’Gorman, N, Syngelaki, A, Paco, Matallana C, Akolekar, R, Cicero, S, Janga, D, Singh, M, Molina, Fs, Persico, N, Jani, Jc, Plasencia, W, Papaioannou, G, Tenenbaum-Gavish, K, Meiri, H, Gizurarson, S, Maclagan, K, Nicolaides, Kh, Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia, New England Journal of Medicine, 377, 613-622, 2017 | No subgroup analysis presented for women with chronic hypertension. Data from secondary publication of this trial (Poon 2017) are included. |
| Rotchell, Y. E., Cruickshank, J. K., Gay, M. P., Griffiths, J., Stewart, A., Farrell, B., Ayers, S., Hennis, A., Grant, A., Duley, L., Collins, R., Barbados Low Dose Aspirin Study in Pregnancy (BLASP): a randomised trial for the prevention of pre-eclampsia and its complications, British Journal of Obstetrics and Gynaecology, 105, 286-92, 1998 | <1% participants had chronic hypertension. |
| Rubin, P. C., Butters, L., Clark, D. M., Reynolds, B., Sumner, D. J., Steedman, D., Low, R. A., Reid, J. L., Placebo-controlled trial of atenolol in treatment of pregnancy-associated hypertension, Lancet, 1, 431-4, 1983 | Women presented with gestational hypertension |
| Rubin,P.C., Butters,L., Low,R.A., Reid,J.L., Atenolol in the treatment of essential hypertension during pregnancy, British Journal of Clinical Pharmacology, 14, 279-281, 1982 | Non randomised trial |
| Sabir, S., Yasmin, S., Abbas, G., Comparison of oral nifedipine with intravenous hydralazine for acute hypertensive emergencies of pregnancy, Journal of Postgraduate Medical Institute, 30, 328-330, 2016 | Women presented with gestational hypertension |
| Schiff, E., Peleg, E., Goldenberg, M., Rosenthal, T., Ruppin, E., Tamarkin, M., Barkai, G., Ben-Baruch, G., Yahal, I., Blankstein, J., Goldman, B., Mashiach, S., The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies, New England Journal of Medicine, 321, 351-356, 1989 | Women with chronic hypertension were excluded. |
| Sharma, C., Soni, A., Gupta, A., Verma, A., Verma, S., Hydralazine vs nifedipine for acute hypertensive emergency in pregnancy: A randomized controlled trial, American Journal of Obstetrics and Gynecology, 2017 | Women with chronic hypertension were excluded |
| Shekhar, S., Gupta, N., Kirubakaran, R., Pareek, P., Oral nifedipine versus intravenous labetalol for severe hypertension during pregnancy: a systematic review and meta-analysis, BJOG: An International Journal of Obstetrics & Gynaecology, 123, 40-7, 2016 | No relevant studies were included |
| Shekhar, S., Sharma, C., Thakur, S., Verma, S., Oral nifedipine or intravenous labetalol for hypertensive emergency in pregnancy: a randomized controlled trial, Obstetrics & Gynecology, 122, 1057-63, 2013 | Women with chronic hypertension were excluded |
| Sibai, B. M., Grossman, R. A., Grossman, H. G., Effects of diuretics on plasma volume in pregnancies with long-term hypertension, American Journal of Obstetrics and Gynecology, 150, 831-835, 1984 | Type or dose of diuretics was not specified |
| Souza, Mesquita Mr, Atallah, An, Bertini, Am, The use of hydralazine and nifedipine as treatment for hypertension emergency during pregnancy, Proceedings of 14th european congress of perinatal medicine;1994 june 5-8; helsinki, finland, Abstract no: 163, 1994 | Abstract |
| Stanescu, A. D., Banica, R., Sima, R. M., Ples, L., Low dose aspirin for preventing fetal growth restriction: A randomised trial, Journal of Perinatal Medicine, 2018 | No data on prevalence of chronic hypertension in participants. |
| Subtil, D, Goeusse, P, Houfflin-Debarge, V, Puech, F, Lequien, P, Breart, G, Uzan, S, Quandalle, F, Delcourt, Ym, Malek, Ym, Randomised comparison of uterine artery Doppler and aspirin (100 mg) with placebo in nulliparous women: the Essai Régional Aspirine Mère-Enfant study (Part 2), Bjog, 110, 485-491, 2003 | Women with chronic hypertension were excluded. |
| Subtil, D, Goeusse, P, Puech, F, Lequien, P, Biausque, S, Breart, G, Uzan, S, Marquis, P, Parmentier, D, Churlet, A, Aspirin (100 mg) used for prevention of pre-eclampsia in nulliparous women: the Essai Régional Aspirine Mère-Enfant study (Part 1), Bjog, 110, 475-484, 2003 | Women with chronic hypertension were excluded. |
| Sureau, C., Prevention of perinatal consequences of pre-eclampsia with low-dose aspirin: Results of the epreda trial, European Journal of Obstetrics Gynecology and Reproductive Biology, 41, 71-73, 1991 | All participants received aspirin. |
| Tewari,S., Kaushish,R., Sharma,S., Gulati,N., Role of low dose aspirin in prevention of pregnancy induced hypertension, Journal of the Indian Medical Association, 95, 43-44, 1997 | No details on inclusion/exclusion of women with chronic hypertension. |
| Trivedi, N. A., A meta-analysis of low-dose aspirin for prevention of preeclampsia, Journal of Postgraduate Medicine, 57, 91-5, 2011 | No subgroup analysis for women with chronic hypertension. |
| Tuimala, R., Hartikainen-Sorri, A. L., Randomized comparison of atenolol and pindolol for treatment of hypertension in pregnancy, Current Therapeutic Research - Clinical and Experimental, 44, 579-584, 1988 | Women presented with gestational hypertension |
| Villa, P. M., Kajantie, E., Raikkonen, K., Pesonen, A. K., Hamalainen, E., Vainio, M., Taipale, P., Laivuori, H., Aspirin in the prevention of pre-eclampsia in high-risk women: A randomised placebo-controlled PREDO Trial and a meta-analysis of randomised trials, BJOG: An International Journal of Obstetrics and Gynaecology, 120, 64-74, 2013 | 16.5% participants had chronic hypertension, but no stratified analysis is reported for these women. |
| Vogel, S. A., Rajaii, R., Ottaviano, G., Kim, L., Yeaton-Massey, A., Caughey, A. B., Low-dose aspirin for prevention of preeclampsia and its complications: A cost effectiveness analysis, Archives of Disease in Childhood: Fetal and Neonatal Edition, 95, 2010 | Conference abstract |
| Voto, Ls, Lapidus, Am, Neira, J, Magulies, M, Treatment of hypertension in pregnancy: atenolol versus alpha-methyldopa, Obstetricia y ginecologia latino-americanas, 43, 335-341, 1985 | Study in Spanish |
| Walker, J. J., Greer, I., Calder, A. A., Treatment of acute pregnancy-related hypertension: Labetalol and hydralazine compared, Postgraduate Medical Journal, 59, 168-170, 1983 | Unclear whether women presented with CHT; only p-values were reported, therefore non abstractable data |
| Walker, K. F., Bugg, G. J., Macpherson, M., McCormick, C., Grace, N., Wildsmith, C., Bradshaw, L., Smith, G. C. S., Thornton, J. G., Randomized trial of labor induction in women 35 years of age or older, New England Journal of Medicine, 374, 813-822, 2016 | Inclusion criteria for the trial covered diferent conditions, and a minority of women presented with hypertension |
| Wallenburg, H. C., Dekker, G. A., Makovitz, J. W., Rotmans, P., Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae, Lancet (London, England), 1, 1-3, 1986 | Trial did not include women with chronic hypertension. |
| Webster, L. M., Conti-Ramsden, F., Seed, P. T., Webb, A. J., Nelson-Piercy, C., Chappell, L. C., Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: A systematic review and meta-analysis, Journal of the American Heart Association, 6, e005526, 2017 | Some of the included studies used antihypertensive medications not relevant for the protocol of this review. The remaining included studies have been considered for inclusion |
| Welt, S. I., Dorminy, J. H., 3rd, Jelovsek, F. R., Crenshaw, M. C., Gall, S. A., The effects of prophylactic management and therapeutics on hypertensive disease in pregnancy: preliminary studies, Obstetrics and Gynecology, 57, 557-65, 1981 | No relevant comparator (hydralazine) |
| Xu, T. T., Zhou, F., Deng, C. Y., Huang, G. Q., Li, J. K., Wang, X. D., Low-Dose Aspirin for Preventing Preeclampsia and Its Complications: A Meta-Analysis, Journal of Clinical Hypertension, 17, 567-73, 2015 | No stratified analysis for women with chronic hypertension. |
Economic studies
Table 17Economic excluded studies with reasons for exclusion
| Study | Reason for exclusion |
|---|---|
| Ahmed RJ, Gafni A, Hutton EK, Hu ZJ, Pullenayegum E, Von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez JJ, Ganzevoort W, Helewa M, Lee SK, Lee T, Logan AG, Moutquin JM, Singer J, Thornton JG, Welch R, Magee LA. The Cost Implications of Less Tight Versus Tight Control of Hypertension in Pregnancy (CHIPS Trial). Hypertension 68(4):1049-1055. 2016 | Not cost-effectiveness analysis. Costs consider Canadian healthcare system and are therefore of limited relevance to UK setting. |
| Barton JR, Istwan NB, Rhea D, Collins A, Stanziano GJ. Cost-savings analysis of an outpatient management program for women with pregnancy-related hypertensive conditions. Dis Manag 9(4):236-41. 2006 | Not cost-effectiveness analysis. Costs considered reflect US healthcare setting therefore of limited relevance to UK. |
| Caughey AB, Sundaram V, Kaimal AJ, Cheng YW, Gienger A, Little SE, Lee JF, Wong L, Shaffer BL, Tran SH, Padula A, McDonald KM, Long EF, Owens DK, Bravata DM. Maternal and neonatal outcomes of elective induction of labor. Evidence report/technology assessment (176) 1-257. 2009 | Not specific to women with chronic hypertension |
| Lai J, Niu B, Caughey AB. A cost-effectiveness analysis on the optimal timing of delivery for women with preeclampsia without severe features. American Journal of Obstetrics and Gynecology, 214(1):S237-S238 2016 | Different population - women with pre-eclampsia |
| Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, Roberts TE, Mol BW, Van der Post JA, Leeflang MM, Barton PM, Hyde CJ, Gupta JK, Khan KS. Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess;12(6). 2008 | Not specific to women with chronic hypertension. |
| Meertens LJE, Scheepers HCJ, Willemse JPMM, Spaanderman MEA, Smits LJM. Should women be advised to use calcium supplements during pregnancy? A decision analysis. Matern Child Nutr 14:e12479. 2018 | Not specific to women with chronic hypertension |
| Merrill M, Aviram A, Niu B, Kuo K, Caughey AB. Tight versus less tight control of blood pressure in pregnant women with chronic hypertension - a cost-effective analysis. American Journal of Obstetrics & Gynecology 214(1):S406-S407 2016 | Available as abstract only (conference poster) |
| O’Mahony JF, Mone F, Tyrrell E, Mulcahy C, McParland P, Breathnach F, Morrison JJ, Higgins J, Daly S, Cotter A, Hunter A, Dicker P, Tully E, Malone FD, Normand C, McAuliffe FM. The cost effectiveness of a policy of universal aspirin versus aspirin indicated by a positive pre-eclampsia screening test. American Journal of Obstetrics & Gynecology 216(1): S483 2017 2016 | Not specific to women with chronic hypertension. |
| Rogozinska E, Marlin N, Jackson L, Rayanagoudar G, Ruifrok AE, Dodds J, Molyneaux E, van Poppel MNM, Poston L, Vinter CA, McAuliffe F, Dodd JM, Owens J, Barakat R, Perales M, Cecatti JG, Surita F, Yeo SA, Bogaerts A, Devlieger R, Teede H, Harrison C, Haakstad L, Shen GX, Shub A, El Beltagy N, Motahari N, Khoury J, Tonstad S, Luoto R, Kinnunen TI, Guelfi K, Facchinetti F, Petrella E, Phelan S, Scudeller TT, Rauh K, Hauner H, Renault K, de Groot CJM, Sagedal LR, Vistad I, Stafne SN, Morkved S, Salvesen KA, Jensen DM, Vitolo M, Astrup A, Geiker NRW, Kerry S, Barton P, Roberts T, Riley RD, Coomarasamy A, Mol BW, Khan KS, Thangaratinam S. Effects of antenatal diet and physical activity on maternal and fetal outcomes: individual patient data meta-analysis and health economic evaluation. Health Technol Assess;21(41) 2017 | Not specific to chronic hypertension |
| van Baaren G‐J, Broekhuijsen K, van Pampus MG, Ganzevoort W, Sikkema JM, Woiski MD, Oudijk MA, Bloemenkamp KWM, Scheepers HCJ, Bremer HA, Rijnders RJP, van Loon AJ, Perquin DAM, Sporken JMJ, Papatsonis DNM, van Huizen ME, Vredevoogd CB, Brons JTJ, Kaplan M, van Kaam AH, Groen H, Porath M, van den Berg PP, Mol BWJ, Franssen MTM, Langenveld J, for the HYPITAT‐II Study Group. An economic analysis of immediate delivery and expectant monitoring in women with hypertensive disorders of pregnancy, between 34 and 37 weeks of gestation (HYPITAT‐II). BJOG 2017;124:453–461 2017 | Not cost-utility analysis. Costs considered reflect US healthcare setting therefore of limited relevance to UK. |
| Vijgen S, Koopmans C, Opmeer B, Groen H, Bijlenga D, Aarnoudse J, Bekedam D, van den Berg P, de Boer K, Burggraaff J, Bloemenkamp K, Drogtrop A, Franx A, de Groot C, Huisjes A, Kwee A, van Loon A, Lub A, Papatsonis D, van der Post J, Roumen F, Scheepers H, Stigter R, Willekes C, Mol B, Van Pampus M. An economic analysis of induction of labour and expectant monitoring in women with gestational hypertension or pre‐eclampsia at term (HYPITAT trial). BJOG, 117: 1577-1585. 2010 | Not specific to women with chronic hypertension |
| Vogel SA, Rajaii R, Ottaviano G, Kim L, Yeaton-Massey A, Caughey AB. Low-dose aspirin for prevention of pre-eclampsia and its complications: a cost-effectiveness analysis. Archives of Disease in Childhood - Fetal and Neonatal Edition;95:Fa46. 2010 | Not specific to women with chronic hypertension. |
| Werner EF, Hauspurg AK, Rouse DJ. A Cost-Benefit Analysis of Low-Dose Aspirin Prophylaxis for the Prevention of Preeclampsia in the United States. Obstet Gynecol 126(6):1242-50 2015 | Not specific to women with chronic hypertension |
| Yeaton-Massey A, Ohno M, Caughey A. Optimal delivery timing for mild gestational hypertension: a decision analysis. American Journal of Obstetrics & Gynecology 210(1): S192 2014 | Different population - mild gestational hypertension not chronic hypertension. |
Appendix L. Research recommendations
1. In women who require treatment for chronic hypertension in pregnancy, what is the effectiveness and safety of antihypertensive agents (compared in head-to-head trials) in improving maternal and perinatal outcomes?
Why this is important
There is a lack of head-to-head evidence comparing the effectiveness and safety of antihypertensive agents in pregnancy. It is not therefor possible to determine the optimal treatment to reduce blood pressure and improve clinical outcomes, while minimising the risk of adverse effects to both the woman and her baby.
Table 18Research recommendation rationale
| Research question | In women who require treatment for chronic hypertension in pregnancy, what is the effectiveness and safety of antihypertensive agents (compared in head-to-head trials) in improving maternal and perinatal outcomes? |
|---|---|
| Importance to ‘patients’ or the population | Use of treatments shown to be effective and safe in pregnancy may reduce the risk of adverse events due to high blood pressure, reduce the burden of monitoring for the woman and reduce the incidence of adverse effects for both the woman and her baby. |
| Relevance to NICE guidance | The committee searched for evidence on this topic but found no high-quality evidence. The committee therefore made the recommendations to consider treatment based on limited available evidence, ensuring that choices of medication take into account pre-existing treatment and the safe use of medicines in pregnancy. However, clinical trials in this area would allow more definitive evidence-based recommendations to be made. |
| Relevance to the NHS | Evidence in this area would lead to better care of women with hypertension in pregnancy, may reduce the need for admission and progression to pre-eclampsia, and lead to better outcomes for both women and their babies (with fewer adverse effects). |
| National priorities | The Department of Health and Social Care Single Departmental Plan (May 2018) aims to reduce variation in health outcomes, and reduce maternal deaths by 20% by 2020 and 50% by 2025. This research recommendation is in response to an identified need in the population. |
| Current evidence base | Lack of evidence; some low or very low quality evidence available. |
| Equality | Pregnant women are entitled to safe pharmacological treatment of their chronic hypertension, without risk to either themselves or their baby |
Table 19Research recommendation modified PICO table
| Criterion | Explanation |
|---|---|
| Population | Women who require treatment for chronic hypertension, including in the first trimester. Setting – hospital-based care. |
| Intervention | Antihypertensive agents, to include labetalol, calcium channel blocker, and to consider use of methyldopa, with specific choice of these and other agents to be justified. |
| Comparator |
|
| Outcome |
|
| Study design | Randomised controlled trial with an internal pilot phase with clear progression criteria to the main trial. |
| Timeframe | Minimum duration of follow-up: To primary discharge of woman and baby. |
2. In women who require treatment for hypertension in pregnancy, what are the adverse neonatal outcomes associated with maternal use of beta-blockers (or mixed alpha-beta blockers)?
There is evidence that beta-blockers and mixed alpha-beta blockers used in pregnancy result in an increased incidence of neonatal hypoglycaemia. However, there is a known transient physiological nadir in glucose levels in well neonates in the immediate postnatal period. It is not clear if the use of beta-blockers/mixed alpha-beta blockers in pregnancy results in a significant decrease in the plasma glucose concentration of a term or preterm neonate, associated with signs and symptoms, resulting in increased hospital length of stay, separation of baby from woman in the immediate postnatal period, or long term adverse outcomes in the baby.
Table 20Research recommendation rationale
| Research question | In women who require treatment for hypertension in pregnancy, what are the adverse neonatal outcomes associated with maternal use of beta-blockers (or mixed alpha/beta-blockers)? |
|---|---|
| Importance to ‘patients’ or the population | Further studies would clarify if beta or mixed alpha/beta-blockers are associated with neonatal hypoglycaemia and may reduce or eliminate the need for invasive heel prick testing to monitor blood glucose in some or all of these babies. |
| Relevance to NICE guidance | The committee searched for evidence on this topic but found no high-quality evidence. Clinical studies in this area would allow more definitive evidence-based recommendations to be made. |
| Relevance to the NHS | Clear recommendations in this area would reduce the likelihood of morbidity and separation of woman and baby in the immediate postnatal period. |
| National priorities | The Department of Health and Social Care Single Departmental Plan (May 2018) aims to reduce the 2010 rate of neonatal deaths and brain injuries in babies that occur during or soon after birth by 20% by 2020 and 50% by 2025 |
| Current evidence base | A systematic review published in 2016 found that there is an increased risk of neonatal blood glucose levels falling below 2.6mmol/L shortly after birth if their mothers received beta blockers or labetalol, a mixed alpha and beta blocker in late pregnancy. However, it is physiological for a newborn’s blood glucose level to fall below this threshold in the immediate postnatal period. This systematic review does not address whether these neonatal blood glucose levels below 2.6mmol/L were associated with any clinical problems or long-term morbidity. |
| Equality | Babies born to women with hypertension in pregnancy are entitled to safe care without risk of long term morbidity. |
Table 21Research recommendation modified PICO table
| Criterion | Explanation |
|---|---|
| Population | Women who require treatment for hypertension. Setting: hospital or community |
| Intervention/Exposure | Maternal use of beta-blocker or mixed alpha/beta-blocker during late pregnancy and peripartum period, with consideration of timing and duration of use. |
| Comparator | Women not using these agents in late pregnancy. |
| Outcome |
Important outcomes: Baby: hypoglycaemia, need for additional treatment for hypoglycaemia, birthweight centile. (Consideration should be given to use of routinely collected data for determination of some outcomes) |
| Study design | A variety of study designs may be suitable, but consideration of a cohort design (with comparator data) should be included. |
| Timeframe | Minimum duration of follow-up: To primary discharge of woman and baby. |
Tables
Table 1Summary of the protocol (PICO table)
| Population | Pregnant women with chronic hypertension. This population includes women with:
|
|---|---|
| Intervention |
|
| Comparison |
|
| Outcome |
Outcomes for babies Critical outcomes:
Critical outcomes:
|
ACE: angiotensin converting enzyme; CP: cerebral palsy; HELLP: haemolysis, elevated liver enzymes, low platelets; MDI: mental development index; mmHg: millimetres of mercury; PDI: psychomotor developmental index; SD: standard deviation
Table 2Summary of the included studies
| Study | Participants/Diagnosis (and definition) | Intervention | Control | Outcomes |
|---|---|---|---|---|
|
Multicentre Individual participant data meta-analysis |
N=3303 women with chronic hypertension No definition provided |
Antiplatelet: predominantly aspirin (27 of the included studies), given in doses ranging from 50 to 150mg per day. 59% of women commenced treatment before 20 weeks’ gestation. 3 trials used aspirin with dipyridamole and 3 used different antiplatelet agents | No intervention: either placebo or no treatment |
|
|
Atallah 1996 (ECPPA) Brazil RCT |
N=473 women with chronic hypertension No definition provided | Aspirin: 60mg PO daily from 12 weeks’ gestation (or immediately following randomisation, if this was after 12 weeks) until delivery | No intervention: placebo tablets daily from 12 weeks’ gestation (or immediately following randomisation, if this was after 12 weeks) until delivery | |
|
UK RCT |
N=29 women with chronic hypertension sBP between 140 and 170mmHg and dBP between 90 and 110mmHg on 2 occasions separated by at least 24 hours | Atenolol: 50mg PO daily up to 200mg | No intervention: placebo tablets |
|
|
Egypt and Saudi Arabia RCT |
N=76 women with chronic hypertension sBP between 140 and 160mmHg and dBP between 90 and 110mmHg at least 6 hours apart in the first half of pregnancy | Induction of labour | Expectant management |
|
|
Brazil RCT |
N=116 women with CHT (90.5%) or previous PE (9.5%) BP ≥ 140/90mmHg diagnosed before pregnancy or before 20 weeks’ gestation | Exercise (30 minutes per week riding a stationary bike) | No intervention |
|
|
South America, North America, Israel, Jordan, Oceania and Europe RCT |
N=981 women with CHT (75.02%) or GH (24.98%) dBP ≥90mmHg before pregnancy or before 20+0 weeks’ gestation | Less-tight control (aiming for a target of dBP of 100mmHg) | Tight control (aiming for a target of dBP of 85mmHg) |
|
|
UK RCT |
N=72 women with CHT (65.2%) or PE (34.8%) BP ≥110/170mmHg on two separate occasions before 20 weeks’ gestational age | Labetalol: 100mg × 4 times/day | Methyldopa: 250 mg × 4 times/day |
|
|
USA RCT |
N=186 women with chronic hypertension Defined as use of antihypertensive agent at baseline, or resting BP ≥ 140/90mmHg on two occasions at least four hours apart prior to pregnancy, or before 20 weeks’ gestation | Aspirin: 60mg PO once daily, started prior to 17 weeks’ gestation | No intervention: placebo tablets started prior to 17 weeks’ gestation | |
|
Italy RCT |
N=240 women with chronic hypertension or nephropathy Defined as diastolic BP 90 to 100mmHg or nephropathy with normal renal function and normal BP | Aspirin: 50mg PO once daily from randomisation (at 16 to 32 weeks) until delivery | No intervention |
|
|
Multicentre (UK, Spain, Italy, Belgium, Greece and Israel) RCT |
N=110 women with chronic hypertension Study participants self-reported a diagnosis of chronic hypertension at the 11-13 week visit | Aspirin: 150mg PO once daily from randomisation (approximately 12-13 weeks) until 36 weeks’ gestation | No intervention: placebo tablet to be taken once daily from randomisation until 36 weeks’ gestation |
|
|
UK RCT |
N=208 women with CHT sBP >140 or dBP>90 on 2 occasions at least 24 hours apart before 28 weeks’ GA | Methyldopa: dose and administration route not reported | No intervention |
|
|
USA RCT |
N=263 women with CHT Definition was not reported |
Methyldopa: 750 mg/day up to 4g/day Labetalol: 300 mg/day increased up to 2400 mg/day. | No intervention |
|
|
Multicentre Individual participant data meta-analysis |
N=2518 women with chronic hypertension No definition provided |
Antiplatelet: predominantly aspirin (15 of the included studies), given in doses ranging from 60 to 150mg per day. 1 trial used aspirin with dipyridamole and 1 used dipyridamole alone. | No intervention: either placebo or no treatment |
|
|
Panama RCT |
N=39 women with CHT BP >140/90 mmHg present before pregnancy or for first time before the 20th week of gestation | Amlodipine: 5mg/day PO | Aspirin: 75mg/day PO |
|
|
Finland RCT |
N=208 women with chronic hypertension (89%) or severe pre-eclampsia in a previous pregnancy CHT defined as BP >140/90mmHg without treatment prior to pregnancy | Aspirin: 50mg aspirin/day PO | No intervention: placebo tablets to be taken daily PO |
|
|
UK RCT |
N=114 women with CHT BP ≥140/90mmHg before 20 weeks’ gestation requiring antihypertensive treatment before 27+ 6 weeks’ | Labetalol: 100 mg BID up to 1800 mg | Nifedipine: 10 mg BID up to 80 mg |
|
|
US RCT |
N=25 women with CHT BP ≥140/90 mmHg on 2 separate occasions at least 6 hours apart | Methyldopa: 250 mg PO TID | No intervention: one placebo tablet PO TID |
|
BID: twice a day; BP: blood pressure; CHT: chronic hypertension; dBP: diastolic blood pressure; GA: gestational age; GH: gestational hypertension; HELLP: haemolysis, elevated liver enzymes and low platelet count; mmHg: millimetres of mercury; N: total number of participants; PE: pre-eclampsia; PO: orally; sBP: systolic blood pressure; TID: three times a day
- a
Data are included in individual participant data meta-analyses (by Askie 2007 or van Vliet 2017) therefore not analysed separately
- b
Participants in this report are also included in the IPD by Askie 2007
FINAL
Evidence reviews
These evidence reviews were developed by The National Guideline Alliance hosted by the Royal College of Obstetricians and Gynaecologists
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.