NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Review question: What interventions for chronic hypertension are effective at improving outcomes for women and infants?
Introduction
Chronic hypertension in pregnancy is hypertension present at the booking visit or before 20 weeks, or if the woman is already taking antihypertensive medication when presenting to maternity services. It can be primary or secondary in aetiology. Its pathophysiology is likely to be different from gestational hypertension, and intervention in chronic hypertension which are successful in reducing complications in the mother and baby may be different from those interventions which improve outcomes in gestational hypertension.
This review will look at the evidence for interventions in chronic hypertension in pregnancy to determine which improve outcomes in the woman and her baby.
Summary of the protocol
See Table 1 for a summary of the population, intervention, comparison and outcome (PICO) characteristics of this review.
Table 1
Summary of the protocol (PICO table).
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual 2014. Methods specific to this review question are described in the review protocol in appendix A.
Declaration of interests were recorded according to NICE’s 2018 conflicts of interest policy (see Register of interests).
Clinical evidence
Included studies
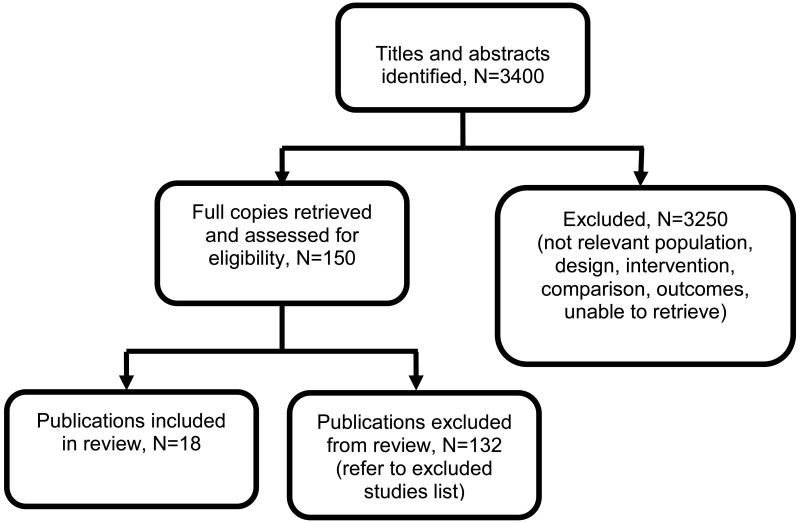
Eighteen articles from 15 randomised controlled trials (RCTs) and 2 individual participant data (IPD) meta-analyses of RCT data have been included in this review (N=5377) (Askie 2007, Atallah 1996, Butters 1990, Cockburn 1982, Hamed 2014, Kasawara 2013, Magee 2015, Moore 1982, Moore 2015, Parazzini 1993, Poon 2017, Redman 1976, Sibai 1990, van Vliet 2017, Vigil-de Gracia 2014, Viinikka 1993, Webster 2017, Weitz 1987).
Summary estimates were reported in the two IPD meta-analyses. However, as the articles did not report the specific data from each of the original studies it was not possible to pool the estimates from the IPD meta-analyses with additional studies. Instead, data from the IPD meta-analyses are presented separately to that from the additional RCTs identified in this review.
See the literature search strategy in appendix B and study selection flow chart in appendix C.
Excluded studies
Studies not included in this review, with reasons for their exclusion, are provided in appendix K.
Summary of clinical studies included in the evidence review
Table 2 provides a brief summary of the included studies.
Table 2
Summary of the included studies.
See appendix D for clinical evidence tables.
Quality assessment of clinical outcomes included in the evidence review
See appendix F for full GRADE tables.
Economic evidence
No economic evidence on the cost effectiveness interventions for chronic hypertension was identified by the systematic search of the economic literature undertaken for this guideline. Economic modelling was not undertaken for this question because other topics were agreed as higher priorities for economic evaluation.
Evidence statements
Comparison 1. Induction of labour versus expectant management
Outcomes for babies
Critical outcomes
Perinatal mortality
- One randomised controlled trial (n=76) provided very low quality evidence to show that there were no clinically important differences in perinatal mortality between those who received induction of labour or expectant management.
Important outcomes
Birth weight
- One randomised controlled trial (n=76) provided low quality evidence to show a clinically important decrease in the weight of babies born of women who received induction of labour compared to those of women who received expectant management.
Gestational age at birth
- One randomised controlled trial (n=76) provided very low quality evidence to show a clinically important decrease in the gestational age at birth for babies born of women who received induction of labour as compared to those of women who received expectant management.
Preterm birth (number of weeks not reported)
- One randomised controlled trial (n=76) provided very low quality evidence to show no clinically important differences in the number of preterm births between those who received induction of labour or expectant management.
Admission to neonatal unit
- One randomised controlled trial (n=76) provided very low quality evidence to show a clinically important increase in the number of babies admitted to a neonatal unit between women who received induction of labour as compared to expectant management.
Outcomes for women
Critical outcomes
Severe hypertension
- One randomised controlled trial (n=76) provided very low quality evidence to show no clinically important difference in the occurrence of severe hypertension between those who received induction of labour or expectant management.
Important outcomes
Superimposed pre-eclampsia
- One randomised controlled trial (n=76) provided very low quality evidence to show no clinically important difference in the incidence of superimposed pre-eclampsia between those who received induction of labour or expectant management.
Placental abruption
- One randomised controlled trial (n=76) provided very low quality evidence to show no clinically important difference in the occurrence of placental abruption between those who received induction of labour or expectant management.
Comparison 2. Exercise versus no intervention
Outcomes for babies
Important outcomes
Birth weight
- One randomised controlled trial (n=109) provided very low quality evidence to show that there were no clinically important differences in birth weight between the babies born of mothers who exercised and those who did not exercise, for weights of <2500g or 2500-3999g. There may be a clinically important reduction in the number of babies born weighing ≥4000g for those who exercise, but there was some uncertainty around the effect (RR 0.43, 95% CI 0.16-1.16).
Admission to neonatal unit
- One randomised controlled trial (n=109) provided very low quality evidence to show no clinically important difference in neonatal unit admission between babies born of mothers who exercised and those who did not exercise.
Outcomes for women
Important outcomes
Mode of birth (caesarean section)
- One randomised controlled trial (n=109) provided very low quality evidence to show no clinically important differences in mode of birth (caesarean section) between women who exercised and those who did not exercise.
Comparison 3. Less-tight versus tight control of blood pressure
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=981) provided very low quality evidence to show no clinically important difference in the occurrence of stillbirth between those who received less-tight or tight control of blood pressure.
Neonatal death
- One randomised controlled trial (n=981) provided very low quality evidence to show no clinically important difference in the occurrence of neonatal death between those who received less-tight or tight control of blood pressure.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=727) provided low quality evidence to show a clinically important decrease in the number of babies born small-for-gestational age for women who received less-tight control of blood pressure, as compared to women who received tight control of blood pressure.
Important outcomes
Birth weight
- One randomised controlled trial (n=981) provided low quality evidence to show no clinically important difference in the birth weight of babies born to women who received less-tight or tight control of blood pressure.
Gestational age at birth
- One randomised controlled trial (n=981) provided low quality evidence to show no clinically important difference in the gestational age at birth for babies born to women who received less-tight or tight control of blood pressure.
Admission to neonatal unit
- One randomised controlled trial (n=959) provided low quality evidence to show no clinically important difference in neonatal unit admissions for babies born to women who received less-tight or tight control of blood pressure.
Outcomes for women
Critical outcomes
Severe hypertension
- One randomised controlled trial (n=732) provided moderate quality evidence to show that less-tight blood pressure control resulted in a clinically important increase in the number of women experiencing severe hypertension, as compared to those with tight control.
Important outcomes
Haemolysis, elevated liver enzymes, low platelets (HELLP)
- One randomised controlled trial (n=981) provided very low quality evidence to show that there may be a clinically important increase in the occurrence of HELLP for those receiving less-tight control, as compared to those receiving tight control, but there was some uncertainty around the estimate (RR 4.45, 95% CI 0.97 to 20.51).
Placental abruption
- One randomised controlled trial (n=981) provided very low quality evidence to show no clinically important difference in the occurrence of placental abruption between those who received less-tight or tight control of blood pressure.
Pre-eclampsia
- One randomised controlled trial (n=731) provided low quality evidence to show no clinically important difference in the occurrence of pre-eclampsia between those who received less-tight or tight control of blood pressure.
Onset of labour (spontaneous onset)
- One randomised controlled trial (n=981) provided very low quality evidence to show no clinically important difference in the number of women experiencing spontaneous onset of labour for those who received less-tight or tight control of blood pressure.
Onset of labour (induced onset)
- One randomised controlled trial (n=981) provided low quality evidence to show no clinically important difference in the number of women experiencing induction of labour for those who received less-tight or tight control of blood pressure.
Onset of labour (elective caesarean)
- One randomised controlled trial (n=981) provided low quality evidence to show no clinically important difference in the occurrence of elective caesarean section for those who received less-tight or tight control of blood pressure.
Mode of birth (caesarean section)
- One randomised controlled trial (n=981) provided low quality evidence to show no clinically important difference in the rate of caesarean section for those who received less-tight or tight control of blood pressure.
Comparison 4. Atenolol versus placebo
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=29) provided very low quality evidence to show no clinically important difference in the occurrence of stillbirth between those who received placebo or atenolol.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=29) provided low quality evidence to show a clinically important increase in the number of babies born small-for-gestational age for those who received atenolol, as compared to those who received placebo.
Important outcomes
Birth weight
- One randomised controlled trial (n=29) provided very low quality evidence to show a clinically important decrease in birth weight for babies of women who received atenolol, as compared to those who received placebo.
Gestational age at birth
- One randomised controlled trial (n=29) provided very low quality evidence to show a mean gestational age of 39.5 weeks for infants born to women taing placebo, and a mean gestational age of 38.5 weeks for infants born to women taking atenolol.
Outcomes for women
Critical outcomes
Blood pressure control
- One randomised controlled trial (n=29) provided very low quality evidence to show a clinically important decrease in diastolic blood pressure for those who received atenolol, as compared to those who received placebo. However, this same study provided very low quality evidence to show that there was no clinically important difference in the systolic blood pressure measurements between those who received atenolol and placebo.
Comparison 5. Labetalol versus no intervention
Outcomes for babies
Critical outcomes
Perinatal death up to 7 days
- One randomised controlled trial (n=176) provided very low quality evidence to show no clinically important difference in perinatal deaths between those who received labetalol or no intervention.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=176) provided very low quality evidence to show that there was no clinically important difference in the number of babies born small-for-gestational age between those who received labetalol or no intervention.
Important outcomes
Preterm birth (<37 weeks)
- One randomised controlled trial (n=176) provided very low quality evidence to show that there was no clinically important difference in preterm birth (<37 weeks) for those who received labetalol or no intervention.
Outcomes for women
Important outcomes
Superimposed pre-eclampsia
- One randomised controlled trial (n=176) provided very low quality evidence to show that there was no clinically important difference in the number of women developing superimposed pre-eclampsia between those who received labetalol or no intervention.
Placental abruption
- One randomised controlled trial (n=176) provided very low quality evidence to show that there was no clinically important difference in the occurrence of placental abruption between those who received labetalol or no intervention.
Mode of birth (caesarean section)
- One randomised controlled trial (n=176) provided very low quality evidence to show that there was no clinically important difference in the number of women undergoing caesarean section between those who received labetalol or no intervention.
Comparison 6. Labetalol versus nifedipine
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the occurrence of stillbirth between those who received labetalol or nifedipine.
Neonatal death up to 7 days
- One randomised controlled trial (n=112) provided moderate quality evidence to show that no neonatal deaths occurred in those who received labetalol or nifedipine.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the number of babies born small-for-gestational age between those who received labetalol or nifedipine.
Important outcomes
Birth weight
- One randomised controlled trial (n=112) provided low quality evidence to show no clinically important difference in the birth weight of babies born to women who received labetalol or nifedipine.
Preterm birth (<37 weeks)
- One randomised controlled trial (n=112) provided low quality evidence to show no clinically important difference in the occurrence of preterm birth (<37 weeks) between those who received labetalol or nifedipine.
Preterm birth (<34 weeks)
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the occurrence of preterm birth (<34 weeks) between those who received labetalol or nifedipine.
Admission to neonatal unit
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the number of babies requiring neonatal unit admission between women who received labetalol or nifedipine.
Gestational age at birth
- One randomised controlled trial (n=112) provided moderate quality evidence to show a clinically important increase in the gestational age at birth for the babies of women who received labetalol compared to women who received nifedipine.
Outcomes for women
Important outcomes
Mode of birth (caesarean section)
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the number of women giving birth by caesarean section between those who received labetalol or nifedipine.
Superimposed pre-eclampsia
- One randomised controlled trial (n=112) provided low quality evidence to show no clinically important difference in the number of women developing superimposed pre-eclampsia between those who received labetalol or nifedipine.
Superimposed pre-eclampsia <34 weeks
- One randomised controlled trial (n=112) provided very low quality evidence to show no clinically important difference in the occurrence of early onset superimposed pre-eclampsia (< 34 weeks) between those who received labetalol or nifedipine.
Eclampsia
- One randomised controlled trial (n=112) provided moderate quality evidence to show no occurrence of eclampsia in women who received labetalol or nifedipine.
Maternal death
- One randomised controlled trial (n=112) provided moderate quality evidence to show that no maternal deaths occurred in those who received labetalol or nifedipine.
Comparison 7. Labetalol versus methyldopa
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=72) provided very low quality evidence to show that no stillbirths occurred in those who received labetalol or methyldopa.
Neonatal death up to 7 days
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no clinically important difference in neonatal death between those who received labetalol or methyldopa.
Small-for-gestational age
- Two randomised controlled trials (n=246) provided very low quality evidence to show no clinically important difference in the number of babies born small-for-gestational age between women who received labetalol or methyldopa.
Important outcomes
Birth weight
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no clinically important difference in infant birth weight between women who received labetalol or methyldopa.
Gestational age at birth
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no clinically important difference in the gestational age at birth for babies born to women who received labetalol or methyldopa.
Admission to neonatal unit
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no difference in the rate of admission to a neonatal unit for babies of women who received labetalol or methyldopa.
Outcomes for women
Critical outcomes
Blood pressure control
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no clinically important difference in the systolic or diastolic blood pressure measurements between those who received labetalol or methyldopa.
Important outcomes
Onset of labour (induction)
- One randomised controlled trial (n=72) provided very low quality evidence to show that there was no clinically important difference in the number of women undergoing induction of labour between those who received labetalol or methyldopa.
Mode of birth (caesarean section)
- Two randomised controlled trials (n=246) provided very low quality evidence to show that there was no clinically important difference in the incidence of caesarean section between those who received labetalol or methyldopa.
Comparison 8. Methyldopa versus placebo
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=25) provided moderate quality evidence to show that no stillbirths occurred in those who received methyldopa or placebo.
Neonatal death
- One randomised controlled trial (n=25) provided moderate quality evidence to show that no neonatal deaths occurred in those who received methyldopa or placebo.
Important outcomes
Gestational age at birth
- One randomised controlled trial (n=25) provided moderate quality evidence to show a clinically important increase in the gestational age at birth for infants of women who received methyldopa compared to those of women who received placebo.
Outcomes for women
Important outcomes
Superimposed pre-eclampsia
- One randomised controlled trial (n=25) provided very low quality evidence to show no clinically important difference in the occurrence of superimposed pre-eclampsia between those who received methyldopa or placebo.
Comparison 9. Methyldopa versus no intervention
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=190) provided very low quality evidence to show a clinically important reduction in stillbirths for those who received methyldopa, compared to no intervention.
Perinatal death
- One randomised controlled trial (n=178) provided very low quality evidence to show no clinically important difference in perinatal death rates between those who received methyldopa or no intervention.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=178) provided very low quality evidence to show that there was no clinically important difference in the number of babies born small-for-gestational age between women who received methyldopa or no intervention.
Important outcomes
Birth weight
- One randomised controlled trial (n=190) provided low quality evidence to show no clinically important difference in the birth weight of babies born to women who received methyldopa or no intervention.
Gestational age at birth
- One randomised controlled trial (n=204) provided low quality evidence to show no clinically important difference in the gestational age of babies born to women who received methyldopa or no intervention.
Preterm birth (<37 weeks)
- One randomised controlled trial (n=178) provided very low quality evidence to show that there was no clinically important difference in preterm births (<37 weeks) between those who received methyldopa or no intervention.
Neurodevelopmental outcomes at ≥ 18 months: impaired vision at 7.5 years old
- One randomised controlled trial (n=190) provided very low quality evidence to show that there may be a clinically important decrease in the number of children with impaired vision at 7.5 years old for those who received methyldopa, as compared to placebo, but there was some uncertainty around the effect (RR 0.47, 95% CI 0.20 to 1.11).
Neurodevelopmental outcomes at ≥ 18 months: impaired hearing at 7.5 years old
- One randomised controlled trial (n=188) provided very low quality evidence to show that there was no clinically important difference in impaired hearing at 7.5 years follow-up between children born to women who received methyldopa or no intervention
Outcomes for women
Important outcomes
Superimposed pre-eclampsia
- One randomised controlled trial (n=178) provided very low quality evidence to show that there was no clinically important difference in the development of superimposed pre-eclampsia between those who received methyldopa or no intervention.
Placental abruption
- One randomised controlled trial (n=178) provided very low quality evidence to show that there was no clinically important difference in the incidence of placental abruption between those who received methyldopa or no intervention
Mode of birth (caesarean section)
- One randomised controlled trial (n=178) provided very low quality evidence to show that there was no clinically important difference in the number of women giving birth by caesarean section between those who received methyldopa or no intervention
Comparison 10. Amlodipine versus aspirin
Outcomes for babies
Critical outcomes
Stillbirth
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the occurrence of stillbirth between those who received amlodipine or aspirin.
Neonatal death
- One randomised controlled trial (n=39) provided moderate quality evidence to show that no neonatal deaths occurred in those who received amlodipine or aspirin.
Small-for-gestational age (birthweight <10th centile)
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the number of babies born small-for-gestational age between those who received amlodipine or aspirin.
Important outcomes
Birth weight
- One randomised controlled trial (n=39) provided low quality evidence to show no clinically important difference in the birth weight of infants born to women who received amlodipine or aspirin.
Preterm birth (weeks not specified)
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the occurrence of preterm birth between those who received amlodipine or aspirin.
Outcomes for women
Critical outcomes
Severe hypertension
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the incidence of severe hypertension between those who received amlodipine or aspirin.
Important outcomes
Placental abruption
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the occurrence of placental abruption between those who received amlodipine or aspirin.
Mode of birth (caesarean section)
- One randomised controlled trial (n=39) provided very low quality evidence to show no clinically important difference in the number of women giving birth by caesarean section between those who received amlodipine or aspirin.
Comparison 11. Aspirin versus no intervention
Outcomes for babies
Critical outcomes
Stillbirth and neonatal death
- Two randomised controlled trials (n=656) provided very low quality evidence to show no clinically important difference in the occurrence of stillbirth and neonatal death between those who received aspirin or no intervention.
Small for gestational age
- Four randomised controlled trials (n=1074) provided very low quality evidence to show no clinically important difference in the number of babies born small-for-gestational age between women who received aspirin or no intervention.
Important outcomes
Birth weight
- One randomised controlled trial (n=197) provided low quality evidence to show no clinically important difference in the birth weight of babies born to women who received aspirin or no intervention.
Gestational age
- One randomised controlled trial (n=197) provided moderate quality evidence to show that there was no clinically important difference in the gestational age at birth of babies born to women who received aspirin or no intervention.
Preterm birth <37 weeks
- Two randomised controlled trials (n=566) provided low quality evidence to show that there was no clinically important difference in in the number of preterm births (<37 weeks) for women who received aspirin, as compared to those who received no intervention
- A meta-analysis of individual participant data from a further 17 RCTs (n=2518) provided moderate quality evidence to show there may be a clinically important reduction in the number of preterm births (<37 weeks) for women who received aspirin, but there was some uncertainty over the estimate (RR 0.73, 95% CI 0.53 to 1.00).
Preterm birth <34 weeks
- A meta-analysis of individual participant data from 17 RCTs (n=2518) provided low quality evidence to show no clinically important difference in the number of preterm births (<34 weeks) between those who received aspirin or no intervention.
Preterm birth <28 weeks
- A meta-analysis of individual participant data from 17 RCTs (n=2518) provided low quality evidence to show no clinically important difference in the number of preterm births (<28 weeks) between those who received aspirin or no intervention.
Admission to neonatal unit
- One randomised controlled trial (n=197) provided low quality evidence to show a clinically important reduction in the number of neonatal unit admissions for babies born to women who received aspirin, as compared to those who received no intervention.
Outcomes for women
Critical outcomes
Severe hypertension
- One randomised controlled trial (n=197) provided very low quality evidence to show no clinically important difference in the occurrence of worsening hypertension between those who received aspirin or no intervention.
- One randomised controlled trial (n=197) provided moderate quality evidence to show no clinically important difference in the diastolic blood pressure at 36 weeks’ gestation between those who received aspirin or no intervention.
Important outcomes
Development of pre-eclampsia
- Two randomised controlled trials (n=307) provided very low quality evidence to show no clinically important difference in the development of pre-eclampsia between those who received aspirin or no intervention.
- A meta-analysis of individual participant data from 31 RCTs (n=3303) provided high quality evidence to show no clinically important difference in the development of pre-eclampsia between those who received aspirin or no intervention.
Spontaneous onset of labour
- One randomised controlled trial (n=197) provided low quality evidence to show no clinically important difference in the number of women who had a spontaneous onset of labour between those who received aspirin or no intervention.
See appendix E for Forest plots.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
Treatment of chronic hypertension in pregnancy aims to control the mother’s blood pressure without leading to any adverse effects on the baby. The committee therefore identified 3 outcomes of critical importance to allow the balance of benefits and harms of interventions to be assessed. These were control of blood pressure (outcome for women) and perinatal mortality (including stillbirth and neonatal death) and small for gestational age (both outcomes for babies).
The committee also identified 7 important outcomes for babies to provide further information on the potential harms to babies. These were birth weight, gestational age at birth, preterm birth (< 28 weeks, <34 weeks, <37 weeks), admission to a neonatal unit, cerebral palsy, neurodevelopmental delay, and neurosensory impairment. Six further important outcomes for women with chronic hypertension were identified, and these were superimposed pre-eclampsia, HELLP, placental abruption, onset of labour, mode of birth, and maternal death.
The quality of the evidence
Eighteen articles were included in the review. The quality of the evidence was assessed with the Cochrane Risk of Bias tool and ranged from moderate to very low. The main sources of potential bias were: lack of information on the randomisation method used, unreported or unclear concealment of allocation, and lack of blinding of participants and investigators.
The committee determined that there was sufficient evidence to allow them to make some recommendations relating to treatment initiation thresholds and treatment targets. However, there was not enough evidence to discriminate between different pharmacologic treatments, therefore they made a research recommendation relating to the choice of pharmacologic agents. There was also concern (based on the committee’s clinical knowledge and expertise) over the potential neonatal adverse outcomes with the use of beta-blockers in women with hypertension, and so the committee made a research recommendation relating to this too.
Benefits and harms
The committee made an overarching recommendation on the advice that should be provided to pregnant women with chronic hypertension, in accordance with existing NICE guidelines on the treatment of hypertension in adults. This guideline does not provide specific advice for pregnant women, but the committee agreed that the principles of treatment and advice (such as exercise and healthy diet) are similar.
No specific evidence was available that demonstrated the blood pressure at which treatment for chronic hypertension should be initiated, but the committee identified that in the CHIPS study (Magee 2015) (which had identified that tight blood pressure control led to a reduced incidence of severe hypertension in women), the treatment threshold had been a diastolic blood pressure of ≥90mmHg. There was no equivalent systolic blood pressure treatment threshold in this study so the committee referred to the NICE guidelines on the treatment of hypertension in adults and used their treatment threshold of ≥140mmHg. Similarly, for the target blood pressure the committee adopted the CHIPS target of ≤85mmHg diastolic and the adult guideline target of ≤135mmHg systolic. There was some low quality evidence that a tighter control of blood pressure may slightly increase the number of babies who were small-for-gestational-age (but with no impact on need for high-level neonatal care or pregnancy loss). However, the committee noted that in the full CHIPS trial (including women with both chronic hypertension and gestational hypertension) no difference was seen in the number of babies who were born small for gestational age, after adjustment for baseline differences between the two groups of participants. Overall, the committee balanced the benefits and harms and made recommendations to adopt these treatment thresholds and treatment targets.
Chronic hypertension is associated with complications during pregnancy, including adverse maternal and neonatal outcomes. However, treatments such as antihypertensives and aspirin also carry potential risks such as side effects for the mother and the possibility of teratogenic effects. Clinicians continuing existing treatment or initiating treatments should inform women of these risks and benefits. There was evidence for beneficial effects on the mother’s blood pressure with tight blood pressure control. There was no evidence of a benefit on placental abruption or preterm birth with any of the interventions, but some evidence for a reduction in stillbirths and increased gestational age at birth with some of the pharmacologic interventions. However, there was also some evidence for harm with interventions – a possible increase in small-for-gestational age babies with tight blood pressure control and atenolol. The committee weighed up the benefits and harms and, based on their clinical expertise as well, agreed that treatment with antihypertensive medication should be continued or initiated in pregnant women with chronic hypertension, in order to reduce the risk of serious complications such as severe hypertension, placental abruption or preterm birth.
The available evidence was not sufficient to recommend one antihypertensive medicine over another as it demonstrated no significant differences between labetalol, nifedipine and methyldopa. The only significant difference noted was a small increase in gestational age at delivery for infants of mothers treated with labetalol, as compared with nifedipine. However, the committee noted that this difference was not seen after adjustment for baseline differences in the treatment groups. When methyldopa was compared with no intervention or placebo, it showed that those who received the active intervention experienced a longer gestational age and fewer stillbirths. The committee discussed the fact that labetalol was specifically licensed in pregnancy (after the 1st trimester) whereas other treatments are not, but that all three medicines had been used in pregnant women for many years with no reports of major adverse effects, had been recommended in the 2010 guideline for gestational hypertension and pre-eclampsia, and that it made sense for clinicians to use the same range of drugs to treat all types of hypertension. The committee therefore chose to recommend labetaolol as the first-line choice due to its licensed status, with nifedipine or methylodopa as alternative treatment options
Aspirin had been included as one of the interventions in the review and there was evidence to show that it may reduce preterm birth (<37 weeks) and neonatal unit admission. The committee therefore chose to retain the recommendation from the previous guideline to use aspirin from the second trimester of pregnancy (12 weeks). The committee noted that the studies used different doses of aspirin, ranging from 50 to 150mg daily, and that common practice in the UK was to offer 75 to 150mg, with there being little evidence to support the optimal dose.
Because of the lack of evidence on the effectiveness and safety of antihypertensives in pregnant women with chronic hypertension, the committee decided to repeat the research recommendation made in the previous version of the guideline, to determine the best agent to use. The committee agreed that as ethnicity has an impact on the choice of antihypertensives outside of pregnancy, this study should include an analysis by different ethnicities.
Labetalol is approved for use in pregnancy, and atenolol had shown some efficacy for blood pressure control but with very limited evidence and possibly some adverse effects. The committee were aware from their own clinical experience and knowledge that these adverse effects included hypoglycaemia, but as there is limited data for both of these medicines, the committee also made a research recommendation to establish whether beta-blockers (and mixed alpha-beta blockers) can be used safely in chronic hypertension in pregnancy.
The committee noted that since the previous guideline had been published, NICE had produced diagnostic guidance on the use of placental growth factor (PlGF) monitoring to help rule-out pre-eclampsia in women between 20+0 and 34+6 weeks. Since chronic hypertension is a risk factor for pre-eclampsia, the committee agreed that a cross-reference to this guidance should be included.
Cost effectiveness and resource use
No relevant studies were identified in a systematic review of the economic evidence.
The committee considered that these recommendations would not lead to an increase in resource use as they reflect standard practice for the majority of centres.
Other factors the committee took into account
The committee were aware of the findings from a recently updated Cochrane systematic review and meta-analysis on antihypertensive treatment in pregnancy, which indicated that beta-blockers and calcium channel blockers were more effective than methyldopa at preventing severe hypertension. The Cochrane review included a mixed population of women with any hypertension during pregnancy and so did not meet the protocol criteria for inclusion in this evidence report (which included women with chronic hypertension only). However, the committee agreed that it would be appropriate to recommend methyldopa as the third-line option, after labetalol and nifedipine, based on the findings of the Cochrane review and their experience of the side-effect profile of methyldopa.
The committee were also aware of 2 forthcoming studies which may provide further evidence in this area. The Chronic Hypertension and Pregnancy (CHAP) study will provide further advice on treatment initiation thresholds (estimated completion date December 2019) and the When to Induce Labour to Limit risk in pregnancy hypertension (WILL) study is investigating the optimal timing of birth.
The committee were aware of a recent publication from NHS England, Saving Babies’ Lives, which recommended the use of low dose aspirin in higher risk women. The dose suggested in this document was 150mg at night, or lower doses (60 to 75 mg) in some circumstances, for example women with hepatic or renal disease. This corresponded with the range of 75mg to 150 mg suggested by the committee.
References
Atallah 1996
Atallah AN, Collins R, Farrell B, Handoll H, Freitas A, Kinsui L, Fukushima O, Amorim M, Eduardo R, Durante A, Vieira C. ECPPA: randomised trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnant women. British Journal of Obstetrics and Gynaecology 1996;103(1):39–47. [PubMed: 8608096]Askie 2007
Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791–8. [PubMed: 17512048]Butters 1990
Butters L, Kennedy S, Rubin PC. Atenolol in essential hypertension during pregnancy. British Medical Journal 1990;301(6752):587–9. [PMC free article: PMC1663720] [PubMed: 2242456]Cockburn 1982
Cockburn J, Ounsted M, Moar VA, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet 1982;319(8273):647–9. [PubMed: 6121965]Hamed 2014
Hamed HO, Alsheeha MA, Abu‐Elhasan AM, Abd Elmoniem AE, Kamal MM. Pregnancy outcomes of expectant management of stable mild to moderate chronic hypertension as compared with planned delivery. International Journal of Gynecology & Obstetrics 2014;127(1):15–20. [PubMed: 24957533]Kasawara 2013
Kasawara KT, Burgos CS, do Nascimento SL, Ferreira NO, Surita FG, Pinto e Silva JL. Maternal and perinatal outcomes of exercise in pregnant women with chronic hypertension and/or previous preeclampsia: a randomized controlled trial. ISRN Obstetrics and Gynecology 2013;doi:10.1155/2013/857047 [PMC free article: PMC3753734] [PubMed: 23997960] [CrossRef]Magee 2015
Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Singer J, Gafni A, Gruslin A. Less-tight versus tight control of hypertension in pregnancy. New England Journal of Medicine 2015;372(5):407–17. [PubMed: 25629739]Moore 1982
Moore MP, Redman CW. The treatment of hypertension in pregnancy. Current Medical Research and Opinion 1982;8(sup1):39–46.Moore 2015
Moore GS, Allshouse AA, Post AL, Galan HL, Heyborne KD. Early initiation of low-dose aspirin for reduction in preeclampsia risk in high-risk women: a secondary analysis of the MFMU High-Risk Aspirin Study. Journal of Perinatology. 2015 May;35(5):328. [PMC free article: PMC4838902] [PubMed: 25474553]Parazzini 1993
Parazzini F, Benedetto C, Frusca T, Gregorini G, Bocciolone L, Marozio L, Romero M, Danesino V, De Gaetano G, Gastaldi A, Massobrio M. Low‐dose aspirin in prevention and treatment of intrauterine growth retardation and pregnancy‐induced hypertension. International Journal of Gynecology & Obstetrics. 1993;43(1):91-.Poon 2017
Poon LC, Wright D, Rolnik DL, Syngelaki A, Delgado JL, Tsokaki T, Leipold G, Akolekar R, Shearing S, De Stefani L, Jani JC. Aspirin for Evidence-Based Preeclampsia Prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. American Journal of Obstetrics & Gynecology 2017;217(5):585–e1. [PubMed: 28784417]Redman 1976
Redman CW, Beilin LJ, Bonnar J, Ounsted MK. Fetal outcome in trial of antihypertensive treatment in pregnancy. Lancet 1976;308(7989):753–6. [PubMed: 61439]Sibai 1990
Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. American Journal of Obstetrics & Gynecology 1990;162(4):960–7. [PubMed: 2183619]van Vliet 2017
van Vliet EO, Askie LA, Mol BW, Oudijk MA. Antiplatelet agents and the prevention of spontaneous preterm birth: a systematic review and meta-analysis. Obstetrics & Gynecology. 2017;129(2):327–36. [PubMed: 28079785]Vigil-De Gracia 2014
Vigil-De Gracia P, Dominguez L, Solis A. Management of chronic hypertension during pregnancy with furosemide, amlodipine or aspirin: a pilot clinical trial. The Journal of Maternal-Fetal & Neonatal Medicine. 2014;27(13):1291–4. [PubMed: 24102416]Viinikka 1993
Viinikka L, Hartikainen‐Sorri AL, Lumme R, Hiilesmaa V, Ylikorkala O. Low dose aspirin in hypertensive pregnant women: effect on pregnancy outcome and prostacyclin‐thromboxane balance in mother and newborn. British Journal of Obstetrics & Gynaecology 1993 100(9):809–15. [PubMed: 8217999]Webster 2017
Webster LM, Myers JE, Nelson-Piercy C, Harding K, Cruickshank JK, Watt-Coote I, Khalil A, Wiesender C, Seed PT, Chappell LC. Labetalol Versus Nifedipine as Antihypertensive Treatment for Chronic Hypertension in Pregnancy: A Randomized Controlled Trial. Hypertension 2017:HYPERTENSIONAHA-117. [PubMed: 28893900]Weitz 1987
Weitz C, Khouzami V, Maxwell K, Johnson JW. Treatment of hypertension in pregnancy with methyldopa: a randomized double blind study. International Journal of Gynecology & Obstetrics 1987;25(1):35–40. [PubMed: 2883043]
Appendices
Appendix A. Review protocol
Appendix B. Literature search strategies
Databases: Medline; Medline EPub Ahead of Print; and Medline In-Process & Other Non-Indexed Citations
Database: Embase; and Embase Classic
Databases: Cochrane Central Register of Controlled Trials; Cochrane Database of Systematic Reviews; Database of Abstracts of Reviews of Effects; and Health Technology Assessment
Health economics search strategies
Databases: Medline; Medline EPub Ahead of Print; and Medline In-Process & Other Non-Indexed Citations
Databases: Embase; and Embase Classic
Database: Cochrane Central Register of Controlled Trials
Databases: Health Technology Assessment; and NHS Economic Evaluation Database
Appendix D. Clinical evidence tables
Table 4. Clinical evidence tables (PDF, 1.0M)
Appendix E. Forest plots
No forest plots were generated for comparisons 1-6 and 8-10, as no meta-analyses were performed
Appendix F. GRADE tables
Table 5. Clinical evidence profile. Comparison 1. Induction of labour versus expectant management
Table 6. Clinical evidence profile. Comparison 2. Exercise versus no intervention
Table 7. Clinical evidence profile. Comparison 3. Less-tight versus tight control of blood pressure
Table 8. Clinical evidence profile. Comparison 4. Atenolol versus placebo
Table 9. Clinical evidence profile. Comparison 5. Labetalol versus no intervention
Table 10. Clinical evidence profile. Comparison 6. Labetalol versus nifedipine
Table 11. Clinical evidence profile. Comparison 7. Labetalol versus methyldopa
Table 12. Clinical evidence profile. Comparison 8. Methyldopa versus placebo
Table 13. Clinical evidence profile. Comparison 9. Methyldopa versus no intervention
Table 14. Clinical evidence profile. Comparison 10. Amlodipine versus aspirin
Appendix H. Economic evidence tables
No economic evidence was identified for this review question.
Appendix I. Health economic evidence profiles
No economic evidence was identified for this review question.
Appendix J. Health economic analysis
No health economic analysis was conducted for this review question.
Appendix K. Excluded studies
Clinical studies
Table 16. Clinical excluded studies with reasons for exclusion
Economic studies
Table 17. Economic excluded studies with reasons for exclusion
Appendix L. Research recommendations
1. In women who require treatment for chronic hypertension in pregnancy, what is the effectiveness and safety of antihypertensive agents (compared in head-to-head trials) in improving maternal and perinatal outcomes?
Why this is important
There is a lack of head-to-head evidence comparing the effectiveness and safety of antihypertensive agents in pregnancy. It is not therefor possible to determine the optimal treatment to reduce blood pressure and improve clinical outcomes, while minimising the risk of adverse effects to both the woman and her baby.
2. In women who require treatment for hypertension in pregnancy, what are the adverse neonatal outcomes associated with maternal use of beta-blockers (or mixed alpha-beta blockers)?
There is evidence that beta-blockers and mixed alpha-beta blockers used in pregnancy result in an increased incidence of neonatal hypoglycaemia. However, there is a known transient physiological nadir in glucose levels in well neonates in the immediate postnatal period. It is not clear if the use of beta-blockers/mixed alpha-beta blockers in pregnancy results in a significant decrease in the plasma glucose concentration of a term or preterm neonate, associated with signs and symptoms, resulting in increased hospital length of stay, separation of baby from woman in the immediate postnatal period, or long term adverse outcomes in the baby.
FINAL
Evidence reviews
These evidence reviews were developed by The National Guideline Alliance hosted by the Royal College of Obstetricians and Gynaecologists
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Chronic hypertension: first-trimester blood pressure control and likelihood of severe hypertension, preeclampsia, and small for gestational age.[Am J Obstet Gynecol. 2018]Chronic hypertension: first-trimester blood pressure control and likelihood of severe hypertension, preeclampsia, and small for gestational age.Nzelu D, Dumitrascu-Biris D, Nicolaides KH, Kametas NA. Am J Obstet Gynecol. 2018 Mar; 218(3):337.e1-337.e7. Epub 2018 Jan 2.
- Perinatal Outcomes in Women With a History of Chronic Hypertension but Normal Blood Pressures Before 20 Weeks of Gestation.[Obstet Gynecol. 2018]Perinatal Outcomes in Women With a History of Chronic Hypertension but Normal Blood Pressures Before 20 Weeks of Gestation.Youngstrom M, Tita A, Grant J, Szychowski JM, Harper LM. Obstet Gynecol. 2018 May; 131(5):827-834.
- Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy.[Cochrane Database Syst Rev. 2019]Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy.Hofmeyr GJ, Manyame S, Medley N, Williams MJ. Cochrane Database Syst Rev. 2019 Sep 16; 9(9):CD011192. Epub 2019 Sep 16.
- Review Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes.[Cochrane Database Syst Rev. 2017]Review Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes.Brown J, Ceysens G, Boulvain M. Cochrane Database Syst Rev. 2017 Jun 22; 6(6):CD012202. Epub 2017 Jun 22.
- Review Different types of dietary advice for women with gestational diabetes mellitus.[Cochrane Database Syst Rev. 2017]Review Different types of dietary advice for women with gestational diabetes mellitus.Han S, Middleton P, Shepherd E, Van Ryswyk E, Crowther CA. Cochrane Database Syst Rev. 2017 Feb 25; 2(2):CD009275. Epub 2017 Feb 25.
- Evidence review for interventions for chronic hypertensionEvidence review for interventions for chronic hypertension
Your browsing activity is empty.
Activity recording is turned off.
See more...